Cell Reports ( IF 7.5 ) Pub Date : 2019-10-22 , DOI: 10.1016/j.celrep.2019.09.032 Kimberly J Cocce 1 , Jeff S Jasper 1 , Taylor K Desautels 1 , Logan Everett 2 , Suzanne Wardell 1 , Thomas Westerling 3 , Robert Baldi 1 , Tricia M Wright 1 , Kendall Tavares 1 , Alex Yllanes 1 , Yeeun Bae 1 , Jeremy T Blitzer 4 , Craig Logsdon 5 , Daniel P Rakiec 6 , David A Ruddy 6 , Tiancong Jiang 7 , Gloria Broadwater 7 , Terry Hyslop 7 , Allison Hall 8 , Muriel Laine 9 , Linda Phung 9 , Geoffrey L Greene 9 , Lesley-Ann Martin 10 , Sunil Pancholi 10 , Mitch Dowsett 11 , Simone Detre 11 , Jeffrey R Marks 12 , Gregory E Crawford 13 , Myles Brown 3 , John D Norris 1 , Ching-Yi Chang 1 , Donald P McDonnell 1
|
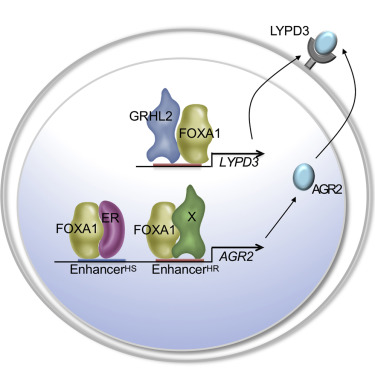
Notwithstanding the positive clinical impact of endocrine therapies in estrogen receptor-alpha (ERα)-positive breast cancer, de novo and acquired resistance limits the therapeutic lifespan of existing drugs. Taking the position that resistance is nearly inevitable, we undertook a study to identify and exploit targetable vulnerabilities that were manifest in endocrine therapy-resistant disease. Using cellular and mouse models of endocrine therapy-sensitive and endocrine therapy-resistant breast cancer, together with contemporary discovery platforms, we identified a targetable pathway that is composed of the transcription factors FOXA1 and GRHL2, a coregulated target gene, the membrane receptor LYPD3, and the LYPD3 ligand, AGR2. Inhibition of the activity of this pathway using blocking antibodies directed against LYPD3 or AGR2 inhibits the growth of endocrine therapy-resistant tumors in mice, providing the rationale for near-term clinical development of humanized antibodies directed against these proteins.
中文翻译:

谱系决定因子 GRHL2 与 FOXA1 合作,建立内分泌治疗耐药乳腺癌的靶向途径。
尽管内分泌疗法对雌激素受体α(ERα)阳性乳腺癌具有积极的临床影响,但新发耐药和获得性耐药限制了现有药物的治疗寿命。鉴于耐药性几乎是不可避免的,我们进行了一项研究,以识别和利用内分泌治疗耐药性疾病中明显的有针对性的脆弱性。使用内分泌治疗敏感和内分泌治疗耐药乳腺癌的细胞和小鼠模型,以及当代的发现平台,我们确定了由转录因子 FOXA1 和 GRHL2、共同调控的靶基因、膜受体 LYPD3 组成的可靶向途径,和 LYPD3 配体 AGR2。使用针对 LYPD3 或 AGR2 的阻断抗体抑制该通路的活性,可抑制小鼠体内内分泌治疗耐药性肿瘤的生长,为针对这些蛋白质的人源化抗体的近期临床开发提供依据。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号