当前位置:
X-MOL 学术
›
J. Alloys Compd.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One-pot synthesis of crossed Fe2O3 nanosheets in-situ grown on Ni foam and the application for H2O2 electrooxidation
Journal of Alloys and Compounds ( IF 5.8 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.jallcom.2019.152770 Congying Song , Guiling Wang , Feifan Zhang , Kai Zhu , Kui Cheng , Ke Ye , Jun Yan , Dianxue Cao , Peng Yan
Journal of Alloys and Compounds ( IF 5.8 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.jallcom.2019.152770 Congying Song , Guiling Wang , Feifan Zhang , Kai Zhu , Kui Cheng , Ke Ye , Jun Yan , Dianxue Cao , Peng Yan
|
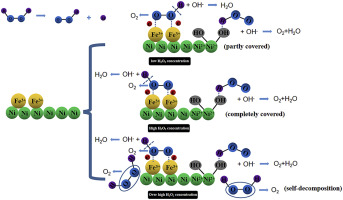
Abstract In this work, one-pot hydrothermal method is used to prepare a novel electrode material of nanosheet-like Fe2O3 in-situ grown on Ni foam. The performance of the as-prepared material toward H2O2 oxidation is investigated systematically. The crossed nanosheet-like structure allows a full contact between the electrode surface and electrolyte which is highly beneficial to the sufficient use of H2O2. The catalytic activity of Fe2O3 grown on Ni foam(Fe2O3/Ni foam) electrode in alkaline medium for H2O2 oxidation is researched by methods of cyclic voltammetry and chronoamperometry. According to the results of cyclic voltammetry in solutions with different H2O2 concentrations, we proposes a possible reaction mechanism that the good ability of Fe3+ to break the oxygen-hydrogen bond in H2O2 leads to the oxidation of H2O2 on the electrode. Meanwhile, Ni foam also has a positive effect on the process of H2O2 oxidation. In a solution of 4 mol L−1 NaOH and 0.4 mol L−1 H2O2, the current density of H2O2 oxidation on the Fe2O3/Ni foam electrode is 800 mA cm−2 revealing a desirable catalytic activity toward H2O2 oxidation. Besides, the activation energy of H2O2 oxidation on the Fe2O3/Ni foam electrode is calculated to be 9.17 kJ mol−1 by studying the influence of temperature on the electrode performance. All the results show that the as-prepared electrode exhibits vast prospect in application.
中文翻译:

在泡沫镍上原位生长的交叉 Fe2O3 纳米片的一锅法合成及其在 H2O2 电氧化中的应用
摘要 本工作采用一锅水热法在泡沫镍上原位生长纳米片状Fe2O3电极材料。系统地研究了所制备材料对 H2O2 氧化的性能。交叉的纳米片状结构允许电极表面和电解质之间完全接触,这非常有利于充分利用 H2O2。采用循环伏安法和计时电流法研究了碱性介质中泡沫镍(Fe2O3/Nifoam)电极上生长的Fe2O3对H2O2氧化的催化活性。根据在不同 H2O2 浓度溶液中循环伏安法的结果,我们提出了可能的反应机制,即 Fe3+ 良好的破坏 H2O2 中氧氢键的能力导致 H2O2 在电极上被氧化。同时,泡沫镍对 H2O2 氧化过程也有积极作用。在 4 mol L-1 NaOH 和 0.4 mol L-1 H2O2 的溶液中,Fe2O3/Ni 泡沫电极上 H2O2 氧化的电流密度为 800 mA cm-2,表明对 H2O2 氧化具有理想的催化活性。此外,通过研究温度对电极性能的影响,计算出 Fe2O3/Ni 泡沫电极上 H2O2 氧化的活化能为 9.17 kJ mol-1。所有结果表明所制备的电极具有广阔的应用前景。通过研究温度对电极性能的影响,计算出 Fe2O3/Ni 泡沫电极上 H2O2 氧化的活化能为 9.17 kJ mol-1。所有结果表明所制备的电极具有广阔的应用前景。通过研究温度对电极性能的影响,计算出 Fe2O3/Ni 泡沫电极上 H2O2 氧化的活化能为 9.17 kJ mol-1。所有结果表明所制备的电极具有广阔的应用前景。
更新日期:2020-03-01
中文翻译:

在泡沫镍上原位生长的交叉 Fe2O3 纳米片的一锅法合成及其在 H2O2 电氧化中的应用
摘要 本工作采用一锅水热法在泡沫镍上原位生长纳米片状Fe2O3电极材料。系统地研究了所制备材料对 H2O2 氧化的性能。交叉的纳米片状结构允许电极表面和电解质之间完全接触,这非常有利于充分利用 H2O2。采用循环伏安法和计时电流法研究了碱性介质中泡沫镍(Fe2O3/Nifoam)电极上生长的Fe2O3对H2O2氧化的催化活性。根据在不同 H2O2 浓度溶液中循环伏安法的结果,我们提出了可能的反应机制,即 Fe3+ 良好的破坏 H2O2 中氧氢键的能力导致 H2O2 在电极上被氧化。同时,泡沫镍对 H2O2 氧化过程也有积极作用。在 4 mol L-1 NaOH 和 0.4 mol L-1 H2O2 的溶液中,Fe2O3/Ni 泡沫电极上 H2O2 氧化的电流密度为 800 mA cm-2,表明对 H2O2 氧化具有理想的催化活性。此外,通过研究温度对电极性能的影响,计算出 Fe2O3/Ni 泡沫电极上 H2O2 氧化的活化能为 9.17 kJ mol-1。所有结果表明所制备的电极具有广阔的应用前景。通过研究温度对电极性能的影响,计算出 Fe2O3/Ni 泡沫电极上 H2O2 氧化的活化能为 9.17 kJ mol-1。所有结果表明所制备的电极具有广阔的应用前景。通过研究温度对电极性能的影响,计算出 Fe2O3/Ni 泡沫电极上 H2O2 氧化的活化能为 9.17 kJ mol-1。所有结果表明所制备的电极具有广阔的应用前景。































 京公网安备 11010802027423号
京公网安备 11010802027423号