当前位置:
X-MOL 学术
›
Nano Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Transcytosis of Nanomedicine for Tumor Penetration.
Nano Letters ( IF 9.6 ) Pub Date : 2019-10-25 , DOI: 10.1021/acs.nanolett.9b03211
Yan Liu 1 , Yingying Huo 2 , Lin Yao 3 , Yawen Xu 2 , Fanqiang Meng 1 , Haifeng Li 1 , Kang Sun 1 , Guangdong Zhou 2, 3 , Daniel S Kohane 4 , Ke Tao 1
Nano Letters ( IF 9.6 ) Pub Date : 2019-10-25 , DOI: 10.1021/acs.nanolett.9b03211
Yan Liu 1 , Yingying Huo 2 , Lin Yao 3 , Yawen Xu 2 , Fanqiang Meng 1 , Haifeng Li 1 , Kang Sun 1 , Guangdong Zhou 2, 3 , Daniel S Kohane 4 , Ke Tao 1
Affiliation
|
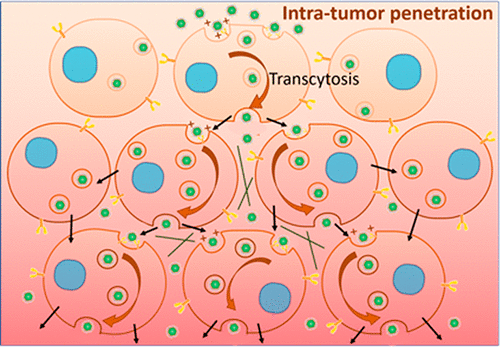
The diffusion of nanomedicines used to treat tumors is severely hindered by the microenvironment, which is a challenge that has emerged as a bottleneck for the effective outcome of nanotherapies. Classical strategies for enhancing tumor penetration rely on passive movement in the extracellular matrix (ECM). Here, we demonstrate that nanomedicine also penetrates tumor lesions via an active trans-cell transportation process. This process was discovered by directly observing the movement of nanoparticles between cells, evaluating the intracellular trafficking pathway of nanoparticles via Rab protein labeling, comparing endocytosis-exocytosis between nanoparticles administered with inhibitors, and correlating the transcytosis process with the micro-CT distribution of nanomedicines. We also demonstrated that enhanced tumor penetration promotes the therapeutic efficacy of a photodynamic therapeutic nanomedicine. Our research thus suggests that transcytosis could be an important positive factor for designing cancer nanomedicines.
中文翻译:

用于肿瘤穿透的纳米药物的胞吞作用。
微环境严重阻碍了用于治疗肿瘤的纳米药物的扩散,这已经成为纳米治疗有效结果的瓶颈,这一挑战已经显现出来。增强肿瘤穿透力的经典策略依赖于细胞外基质(ECM)的被动运动。在这里,我们证明了纳米药物还可以通过主动的跨细胞转运过程穿透肿瘤病变。通过直接观察纳米粒子在细胞之间的运动,通过Rab蛋白标记评估纳米粒子的细胞内运输途径,比较使用抑制剂的纳米粒子之间的胞吞作用-胞吐作用以及将胞吞作用过程与纳米药物的微CT分布相关联,发现了这一过程。我们还证明增强的肿瘤渗透促进了光动力治疗性纳米药物的治疗功效。因此,我们的研究表明,胞吞作用可能是设计癌症纳米药物的重要积极因素。
更新日期:2019-10-25
中文翻译:

用于肿瘤穿透的纳米药物的胞吞作用。
微环境严重阻碍了用于治疗肿瘤的纳米药物的扩散,这已经成为纳米治疗有效结果的瓶颈,这一挑战已经显现出来。增强肿瘤穿透力的经典策略依赖于细胞外基质(ECM)的被动运动。在这里,我们证明了纳米药物还可以通过主动的跨细胞转运过程穿透肿瘤病变。通过直接观察纳米粒子在细胞之间的运动,通过Rab蛋白标记评估纳米粒子的细胞内运输途径,比较使用抑制剂的纳米粒子之间的胞吞作用-胞吐作用以及将胞吞作用过程与纳米药物的微CT分布相关联,发现了这一过程。我们还证明增强的肿瘤渗透促进了光动力治疗性纳米药物的治疗功效。因此,我们的研究表明,胞吞作用可能是设计癌症纳米药物的重要积极因素。

































 京公网安备 11010802027423号
京公网安备 11010802027423号