当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A comprehensive study of the red persistent luminescence mechanism of Y2O2S:Eu,Ti,Mg†
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2019-10-22 , DOI: 10.1039/c9cp04622d Bingyan Qu 1, 2, 3, 4 , Juntao Wang 1, 2, 3, 4 , Kai Liu 1, 2, 3, 4 , Rulong Zhou 1, 2, 3, 4 , Lei Wang 1, 2, 3, 4
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2019-10-22 , DOI: 10.1039/c9cp04622d Bingyan Qu 1, 2, 3, 4 , Juntao Wang 1, 2, 3, 4 , Kai Liu 1, 2, 3, 4 , Rulong Zhou 1, 2, 3, 4 , Lei Wang 1, 2, 3, 4
Affiliation
|
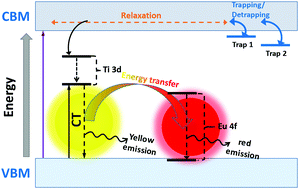
Y2O2S:Eu,Ti,Mg, a persistent luminescence (PersL) material that exhibits eye-sensitive red emission for longer than 4–5 h, has attracted much attention and has been intensively researched over the past decade. If it is figured out how to prolong its decay time for longer than 8 h, the amazing candle-like red PersL performance, once lit, can illuminate a room all night without electricity. However, the PersL mechanism is still confusing, since different investigators have their own unique understanding about it based on their personal experimental observations. In this work, a more comprehensive and detailed investigation of the PersL mechanism is carried out, based on the defect levels induced by Eu, Ti, and Mg impurities and anion vacancies, using first-principles calculations. Our calculated results suggest that the empty spin-down 4f levels of Eu3+ appear in the band gap, while the occupied spin-up 4f levels are just below the valence band maximum (VBM). The 3d levels of Ti4+ are located in the band gap, with the highest levels around 1.4 eV below the conduction band minimum (CBM). Positively charged anion vacancies were found to induce empty defect levels just below the CBM and so could serve as electron trap centers, which prolong the lifetimes of excited electrons and lead to the PersL of the Ti4+ ion. When Eu3+ is co-doped with Ti4+, the energy of the excited Ti4+ ions is transferred to Eu3+. This mechanism can explain well most of the experimental observations that have appeared in the literature over the past decade. The obtained PersL mechanism is very clear in terms of the roles played by most types of defect, so we hope it can provide physical understanding and create intrigue around the idea of practical guidelines for the design of new red PersL materials in the future.
中文翻译:

Y的红色持续发光机理的综合研究2 Ø 2 S:Eu的,钛,镁†
Y 2 O 2S:Eu,Ti,Mg是一种持续发光(PersL)的材料,其呈现出对眼睛敏感的红色发光时间超过4-5小时,因此备受关注,并且在过去十年中进行了深入研究。如果弄清楚如何将其衰变时间延长到8小时以上,那么令人惊叹的蜡烛般的红色PersL性能一旦亮起,就可以整夜不用电照亮整个房间。但是,PersL机制仍然令人困惑,因为不同的研究人员基于他们的个人实验观察,对此有自己的独特理解。在这项工作中,使用第一性原理,基于Eu,Ti和Mg杂质以及阴离子空位引起的缺陷水平,对PersL机理进行了更全面,更详细的研究。我们的计算结果表明,空的自旋下降4f的Eu3+出现在带隙中,而占用的向上旋转4f电平恰好在价带最大值(VBM)之下。Ti 4+的3d能级位于带隙中,最高能级比导带最小值(CBM)低1.4 eV左右。发现带正电荷的阴离子空位会在CBM下方引发空缺的缺陷水平,因此可以用作电子陷阱中心,从而延长了激发电子的寿命并导致Ti 4+离子的PersL。当Eu 3+与Ti 4+共掺杂时,激发的Ti 4+离子的能量转移到Eu 3+上。。这种机制可以很好地解释过去十年来文献中出现的大多数实验观察结果。就大多数类型的缺陷所起的作用而言,所获得的PersL机制非常清楚,因此我们希望它可以提供物理理解,并为将来设计新的红色PersL材料的实用准则的思想带来启发。
更新日期:2019-11-20
中文翻译:

Y的红色持续发光机理的综合研究2 Ø 2 S:Eu的,钛,镁†
Y 2 O 2S:Eu,Ti,Mg是一种持续发光(PersL)的材料,其呈现出对眼睛敏感的红色发光时间超过4-5小时,因此备受关注,并且在过去十年中进行了深入研究。如果弄清楚如何将其衰变时间延长到8小时以上,那么令人惊叹的蜡烛般的红色PersL性能一旦亮起,就可以整夜不用电照亮整个房间。但是,PersL机制仍然令人困惑,因为不同的研究人员基于他们的个人实验观察,对此有自己的独特理解。在这项工作中,使用第一性原理,基于Eu,Ti和Mg杂质以及阴离子空位引起的缺陷水平,对PersL机理进行了更全面,更详细的研究。我们的计算结果表明,空的自旋下降4f的Eu3+出现在带隙中,而占用的向上旋转4f电平恰好在价带最大值(VBM)之下。Ti 4+的3d能级位于带隙中,最高能级比导带最小值(CBM)低1.4 eV左右。发现带正电荷的阴离子空位会在CBM下方引发空缺的缺陷水平,因此可以用作电子陷阱中心,从而延长了激发电子的寿命并导致Ti 4+离子的PersL。当Eu 3+与Ti 4+共掺杂时,激发的Ti 4+离子的能量转移到Eu 3+上。。这种机制可以很好地解释过去十年来文献中出现的大多数实验观察结果。就大多数类型的缺陷所起的作用而言,所获得的PersL机制非常清楚,因此我们希望它可以提供物理理解,并为将来设计新的红色PersL材料的实用准则的思想带来启发。































 京公网安备 11010802027423号
京公网安备 11010802027423号