当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Hydrogenolysis of Glycerol to 1,3-Propanediol over Rhenium-Oxide-Modified Iridium Nanoparticles Coating Rutile Titania Support
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-11-04 , DOI: 10.1021/acscatal.9b03824 Lujie Liu 1 , Takehiro Asano 1 , Yoshinao Nakagawa 1, 2 , Masazumi Tamura 1, 2 , Kazu Okumura 3 , Keiichi Tomishige 1, 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-11-04 , DOI: 10.1021/acscatal.9b03824 Lujie Liu 1 , Takehiro Asano 1 , Yoshinao Nakagawa 1, 2 , Masazumi Tamura 1, 2 , Kazu Okumura 3 , Keiichi Tomishige 1, 2
Affiliation
|
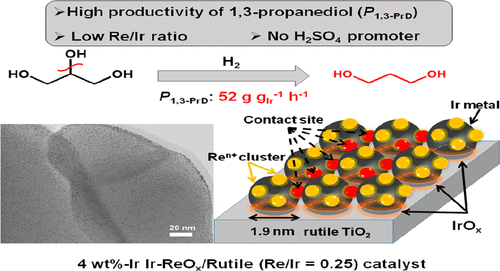
The effect of support in Ir-ReOx catalysts for glycerol hydrogenolysis to 1,3-propanediol was investigated. Rutile TiO2 support showed high activity, even higher than previously reported SiO2 support. Anatase TiO2, C, ZrO2, CeO2, Al2O3, and MgO supports showed very low activity of supported Ir-ReOx pairs. Higher Ir-based 1,3-propanediol productivity of Ir-ReOx/rutile catalyst was obtained at the initial stage even with lower Re/Ir ratio (typical Ir loading amount, 4 wt %, nominal ratio of 0.25; actual ratio of 0.24) without addition of H2SO4 than that of Ir-ReOx/SiO2. The 1,3-propanediol productivity over Ir-ReOx catalysts showed dependency on catalyst compositions (metal loading amount), and the relationship between catalyst structure and activity was further established over Ir-ReOx/rutile. Relatively high Ir loading amount in comparison with small surface area (6 wt %, on 6 m2 g–1 rutile TiO2) showed the highest activity (Ir-based activity). From combined characterization results altogether (TPR, TEM, XPS, XAS, CO adsorption, CO FT-IR) with a kinetics study, the Ir metal particles interacted with the partially oxidized ReOx cluster (average valence of Re: +3) almost totally covering the surface of rutile TiO2 particles, and the active site was the Ir-ReOx interface. Small amounts of Ir species (∼20%) were incompletely reduced; however, such IrOx species as well as rutile TiO2 support were not directly involved in glycerol hydrogenolysis. The role of rutile support was regarded as providing a unique environment for stabilization of uniform and small Ir-ReOx particles with very high surface density on rutile TiO2, which increased the number of active sites per Re amount.
中文翻译:

金红石型二氧化钛载体上氧化R修饰的铱纳米粒子对甘油的选择性加氢水解成1,3-丙二醇
研究了Ir-ReO x催化剂中的载体对甘油氢解为1,3-丙二醇的影响。金红石型TiO 2载体显示出高活性,甚至高于以前报道的SiO 2载体。锐钛矿型TiO 2,C,ZrO 2,CeO 2,Al 2 O 3和MgO载体显示出极低的负载型Ir-ReO x对活性。即使在较低的Re / Ir比(典型的Ir加载量,4 wt%,标称比为0.25;实际比为0.24)下,在初始阶段仍可获得较高的Ir-ReO x /金红石催化剂的基于Ir的1,3-丙二醇生产率),而无需添加H 2 SO 4比Ir-ReO x / SiO 2高。Ir-ReO x催化剂上的1,3-丙二醇生产率显示出对催化剂组成(金属负载量)的依赖性,并且在Ir-ReO x /金红石上进一步建立了催化剂结构与活性之间的关系。与较小的表面积(6 wt%,在6 m 2 g –1金红石型TiO 2上)相比,较高的Ir负载量显示出最高的活性(基于Ir的活性)。通过结合表征结果(TPR,TEM,XPS,XAS,CO吸附,CO FT-IR)和动力学研究,Ir金属颗粒与部分氧化的ReO x相互作用团簇(Re的平均价:+3)几乎完全覆盖了金红石型TiO 2颗粒的表面,并且活性部位是Ir-ReO x界面。少量的Ir种类(〜20%)未完全还原;然而,这种IrO x物种以及金红石TiO 2载体并不直接参与甘油的氢解。金红石载体的作用被认为为在金红石TiO 2上稳定具有很高表面密度的均匀且小的Ir-ReO x颗粒提供了独特的环境,从而增加了每Re含量的活性位点数量。
更新日期:2019-11-04
中文翻译:

金红石型二氧化钛载体上氧化R修饰的铱纳米粒子对甘油的选择性加氢水解成1,3-丙二醇
研究了Ir-ReO x催化剂中的载体对甘油氢解为1,3-丙二醇的影响。金红石型TiO 2载体显示出高活性,甚至高于以前报道的SiO 2载体。锐钛矿型TiO 2,C,ZrO 2,CeO 2,Al 2 O 3和MgO载体显示出极低的负载型Ir-ReO x对活性。即使在较低的Re / Ir比(典型的Ir加载量,4 wt%,标称比为0.25;实际比为0.24)下,在初始阶段仍可获得较高的Ir-ReO x /金红石催化剂的基于Ir的1,3-丙二醇生产率),而无需添加H 2 SO 4比Ir-ReO x / SiO 2高。Ir-ReO x催化剂上的1,3-丙二醇生产率显示出对催化剂组成(金属负载量)的依赖性,并且在Ir-ReO x /金红石上进一步建立了催化剂结构与活性之间的关系。与较小的表面积(6 wt%,在6 m 2 g –1金红石型TiO 2上)相比,较高的Ir负载量显示出最高的活性(基于Ir的活性)。通过结合表征结果(TPR,TEM,XPS,XAS,CO吸附,CO FT-IR)和动力学研究,Ir金属颗粒与部分氧化的ReO x相互作用团簇(Re的平均价:+3)几乎完全覆盖了金红石型TiO 2颗粒的表面,并且活性部位是Ir-ReO x界面。少量的Ir种类(〜20%)未完全还原;然而,这种IrO x物种以及金红石TiO 2载体并不直接参与甘油的氢解。金红石载体的作用被认为为在金红石TiO 2上稳定具有很高表面密度的均匀且小的Ir-ReO x颗粒提供了独特的环境,从而增加了每Re含量的活性位点数量。































 京公网安备 11010802027423号
京公网安备 11010802027423号