当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cytosolic Delivery of Macromolecules in Live Human Cells Using the Combined Endosomal Escape Activities of a Small Molecule and Cell Penetrating Peptides.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2019-10-31 , DOI: 10.1021/acschembio.9b00585 Jason Allen 1 , Kristina Najjar 1 , Alfredo Erazo-Oliveras 1 , Helena M Kondow-McConaghy 1 , Dakota J Brock 1 , Kristin Graham 1 , Elizabeth C Hager 1 , Andrea L J Marschall 2 , Stefan Dübel 2 , Rudolph L Juliano 3 , Jean-Philippe Pellois 1, 4
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2019-10-31 , DOI: 10.1021/acschembio.9b00585 Jason Allen 1 , Kristina Najjar 1 , Alfredo Erazo-Oliveras 1 , Helena M Kondow-McConaghy 1 , Dakota J Brock 1 , Kristin Graham 1 , Elizabeth C Hager 1 , Andrea L J Marschall 2 , Stefan Dübel 2 , Rudolph L Juliano 3 , Jean-Philippe Pellois 1, 4
Affiliation
|
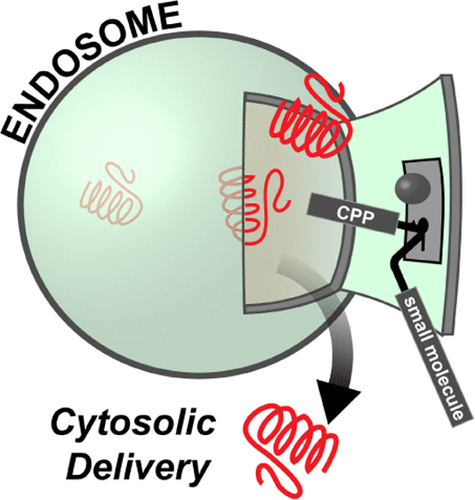
Ineffective cellular delivery is a common problem in numerous biological applications. Developing delivery reagents that work robustly in a variety of experimental settings remains a challenge. Herein, we report how peptides derived from the prototypical cell penetrating peptide TAT can be used in combination with a small molecule, UNC7938, to deliver macromolecules into the cytosol of cells by a simple co-incubation protocol. We establish successful delivery of peptides, DNA plasmids, and a single-chain variable fragment antibody. We also demonstrate that delivery works in hard-to-transfect mammalian cells and under conditions typically inhibitory to cell-penetrating peptides. Mechanistically, UNC7938 destabilizes the membrane of endosomes. This, in turn, enhances the endosome-leakage activity of cell-penetrating peptides and facilitates the endosomal escape of macromolecules initially internalized by mammalian cells via endocytosis. This combined selective membrane-destabilization represents a new chemical space for delivery tools and provides a novel solution to the problem of endosomal entrapment that often limits the effectiveness of reagent-based delivery approaches.
中文翻译:

使用小分子和细胞穿透肽的结合的内体逸出活性,在活的人类细胞中大分子的胞质传递。
在许多生物学应用中,无效的细胞递送是一个普遍的问题。开发在各种实验条件下都能稳定发挥作用的递送试剂仍然是一个挑战。本文中,我们报告了如何将源自典型细胞穿透肽TAT的肽与小分子UNC7938结合使用,以通过简单的共孵育方案将大分子递送到细胞的细胞质中。我们建立了肽,DNA质粒和单链可变片段抗体的成功交付方式。我们还证明了在难以转染的哺乳动物细胞中以及通常在抑制细胞穿透性肽的条件下可以进行递送。从机理上讲,UNC7938破坏了内体的膜。反过来,增强了细胞穿透肽的内体泄漏活性,并促进了最初被哺乳动物细胞内吞作用内化的大分子的内体逃逸。这种组合的选择性膜去稳定化为递送工具代表了一个新的化学领域,并为通常限制基于试剂的递送方法的有效性的内体截留问题提供了一种新颖的解决方案。
更新日期:2019-11-01
中文翻译:

使用小分子和细胞穿透肽的结合的内体逸出活性,在活的人类细胞中大分子的胞质传递。
在许多生物学应用中,无效的细胞递送是一个普遍的问题。开发在各种实验条件下都能稳定发挥作用的递送试剂仍然是一个挑战。本文中,我们报告了如何将源自典型细胞穿透肽TAT的肽与小分子UNC7938结合使用,以通过简单的共孵育方案将大分子递送到细胞的细胞质中。我们建立了肽,DNA质粒和单链可变片段抗体的成功交付方式。我们还证明了在难以转染的哺乳动物细胞中以及通常在抑制细胞穿透性肽的条件下可以进行递送。从机理上讲,UNC7938破坏了内体的膜。反过来,增强了细胞穿透肽的内体泄漏活性,并促进了最初被哺乳动物细胞内吞作用内化的大分子的内体逃逸。这种组合的选择性膜去稳定化为递送工具代表了一个新的化学领域,并为通常限制基于试剂的递送方法的有效性的内体截留问题提供了一种新颖的解决方案。































 京公网安备 11010802027423号
京公网安备 11010802027423号