当前位置:
X-MOL 学术
›
J. Pharmaceut. Biomed. Anal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pharmacokinetic and metabolic profiling studies of sennoside B by UPLC-MS/MS and UPLC-Q-TOF-MS.
Journal of Pharmaceutical and Biomedical Analysis ( IF 3.1 ) Pub Date : 2019-10-21 , DOI: 10.1016/j.jpba.2019.112938 Hongyu Wu 1 , Feng Feng 1 , Xiunan Jiang 1 , Binchuan Hu 1 , Jieying Qiu 1 , CaiHong Wang 1 , Zheng Xiang 1
Journal of Pharmaceutical and Biomedical Analysis ( IF 3.1 ) Pub Date : 2019-10-21 , DOI: 10.1016/j.jpba.2019.112938 Hongyu Wu 1 , Feng Feng 1 , Xiunan Jiang 1 , Binchuan Hu 1 , Jieying Qiu 1 , CaiHong Wang 1 , Zheng Xiang 1
Affiliation
|
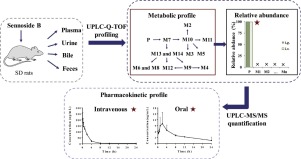
Sennoside B is a specific dianthrone compound extracted from senna, which is widely used as a stimulant laxative but has potential side effects. This study aimed to obtain the metabolic and pharmacokinetic data of sennoside B. The metabolic profiles of sennoside B were obtained from rat plasma, urine, bile and feces by an ultra-performance liquid chromatography quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF-MS). As a result, 14 metabolites were structurally identified and the proposed metabolic pathways of sennoside B included hydrolysis to aglycones, release of rhein-type anthrone, and extensive conjugation. As the only compound detected in the plasma samples after intravenous and intragastric administrations, the prototype was selected as the plasma marker in the pharmacokinetic study. A simple and sensitive ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) method was developed for the quantitation of sennoside B in rat plasma. The linear range of sennoside B was 5-1000 ng/mL (R2 ≥ 0.991) and the lowest limit of quantification (LLOQ) was 5 ng/mL. The intra- and inter- precisions of the assay were less than 10%, whereas accuracy ranged from 85.80% to 103.80%. The extraction recovery, matrix effect and stability of sennoside B were within acceptable limits. The established method was well validated and successfully applied to the pharmacokinetic study of sennoside B. The oral absolute bioavailability of sennoside B was calculated as 3.60% and the value apparent volume of distribution of intravenous and intragastric administrations were 32.47 ± 10.49 L/kg and 7646 ± 1784 L/kg, respectively. The maximum plasma concentrations were 212.6 ± 50.9 μg/L and 14.06 ± 2.73 μg/L for intravenous and intragastric dosing groups, respectively. According to the current results of pharmacokinetic and metabolic profiling studies, metabolites with high abundance in tissues would be the next object in the pharmacokinetic study of sennoside B.
中文翻译:

通过UPLC-MS / MS和UPLC-Q-TOF-MS对森诺甙B进行药代动力学和代谢谱分析。
番泻苷B是从番泻叶中提取的一种特定的二蒽酮化合物,被广泛用作刺激性泻药,但具有潜在的副作用。这项研究旨在获得森诺甙B的代谢和药代动力学数据。通过超高效液相色谱四极杆飞行时间质谱(UPLC-Q)从大鼠血浆,尿液,胆汁和粪便获得森诺甙B的代谢谱-TOF-MS)。结果,在结构上鉴定了14种代谢物,并且拟议的番泻苷B代谢途径包括水解为糖苷配基,释放大黄酸型蒽酮和广泛的结合。作为静脉和胃内给药后血浆样品中唯一检测到的化合物,原型被选为药代动力学研究中的血浆标志物。建立了一种简单灵敏的超高效液相色谱-串联质谱法(UPLC-MS / MS),用于定量测定大鼠血浆中的番石榴苷B。番泻苷B的线性范围为5-1000 ng / mL(R2≥0.991),最低定量限(LLOQ)为5 ng / mL。测定的内部和内部精度小于10%,而精度范围为85.80%至103.80%。番石榴苷B的提取回收率,基质效应和稳定性均在可接受的范围内。所建立的方法得到了很好的验证,并成功地应用于了番泻苷B的药代动力学研究。计算出的番泻苷B的口服绝对生物利用度为3.60%,静脉内和胃内给药的表观分布体积值为32.47±10.49 L / kg和7646分别为±1784 L / kg。静脉和胃内给药组的最大血浆浓度分别为212.6±50.9μg/ L和14.06±2.73μg/ L。根据目前的药代动力学和代谢谱研究的结果,组织中高丰度的代谢产物将成为Sennoside B药代动力学研究的下一个目标。
更新日期:2019-10-22
中文翻译:

通过UPLC-MS / MS和UPLC-Q-TOF-MS对森诺甙B进行药代动力学和代谢谱分析。
番泻苷B是从番泻叶中提取的一种特定的二蒽酮化合物,被广泛用作刺激性泻药,但具有潜在的副作用。这项研究旨在获得森诺甙B的代谢和药代动力学数据。通过超高效液相色谱四极杆飞行时间质谱(UPLC-Q)从大鼠血浆,尿液,胆汁和粪便获得森诺甙B的代谢谱-TOF-MS)。结果,在结构上鉴定了14种代谢物,并且拟议的番泻苷B代谢途径包括水解为糖苷配基,释放大黄酸型蒽酮和广泛的结合。作为静脉和胃内给药后血浆样品中唯一检测到的化合物,原型被选为药代动力学研究中的血浆标志物。建立了一种简单灵敏的超高效液相色谱-串联质谱法(UPLC-MS / MS),用于定量测定大鼠血浆中的番石榴苷B。番泻苷B的线性范围为5-1000 ng / mL(R2≥0.991),最低定量限(LLOQ)为5 ng / mL。测定的内部和内部精度小于10%,而精度范围为85.80%至103.80%。番石榴苷B的提取回收率,基质效应和稳定性均在可接受的范围内。所建立的方法得到了很好的验证,并成功地应用于了番泻苷B的药代动力学研究。计算出的番泻苷B的口服绝对生物利用度为3.60%,静脉内和胃内给药的表观分布体积值为32.47±10.49 L / kg和7646分别为±1784 L / kg。静脉和胃内给药组的最大血浆浓度分别为212.6±50.9μg/ L和14.06±2.73μg/ L。根据目前的药代动力学和代谢谱研究的结果,组织中高丰度的代谢产物将成为Sennoside B药代动力学研究的下一个目标。






























 京公网安备 11010802027423号
京公网安备 11010802027423号