Bioorganic & Medicinal Chemistry Letters ( IF 2.5 ) Pub Date : 2019-10-21 , DOI: 10.1016/j.bmcl.2019.126749
Ryo Mizojiri 1 , Moriteru Asano 1 , Masako Sasaki 1 , Yoshihiko Satoh 1 , Yukiko Yamamoto 1 , Hiroyuki Sumi 1 , Hironobu Maezaki 1
|
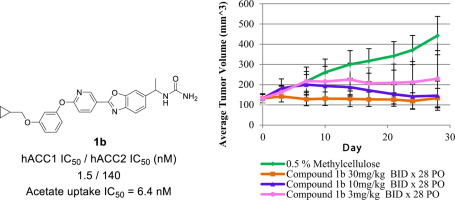
In our effort to explore the potential of ACC1-selective inhibitor as in vivo probe molecule, a series of 1,3-benzoxazole derivatives was synthesized. Previously, we reported a series of novel bicyclic and monocyclic ACC1-selective inhibitors. Among them, compound 1a exhibited highly potent cellular activity (acetate uptake IC50 = 0.76 nM) as well as promising in vivo PD efficacy. However, compound 1a caused severe body weight reduction in repeated dose administration in the mouse model. Since 1a showed potent inhibitory activity against mouse ACC1 as well as strong inhibition of mouse ACC2, we further examined a series of 1a analogues in order to reduce undesirable body weight change. The replacement of acetamide moiety with ureido moiety dramatically improved selectivity of mouse ACC1 against ACC2. In addition, analogue 1b displayed favorable bioavailability in mouse cassette dosing PK study, hence in vivo PD studies were also carried out. Oral administration of 1b significantly reduced the concentration of malonyl-CoA in HCT-116 xenograft tumors at doses of more than 30 mg/kg. Furthermore, compound 1b showed significant antitumor efficacy in 786-O xenograft mice at an oral dose of 30 mg/kg (T/C = 0.5%). Accordingly, our novel potent ACC1-selective inhibitor represents a set of useful orally-available research tools, as well as potential therapeutic agents particularly in terms of new cancer therapies.
中文翻译:

有效,选择性和口服可用的ACC1抑制剂的鉴定和药理评估。
为了探索ACC1选择性抑制剂作为体内探针分子的潜力,我们合成了一系列1,3-苯并恶唑衍生物。以前,我们报道了一系列新型的双环和单环ACC1选择性抑制剂。其中,化合物1a表现出高度有效的细胞活性(醋酸盐吸收IC 50 = 0.76 nM)以及有希望的体内PD功效。但是,化合物1a在小鼠模型中的重复剂量给药中导致严重的体重减轻。由于1a对小鼠ACC1表现出有效的抑制活性以及对小鼠ACC2的强抑制作用,因此我们进一步研究了一系列1a类似物以减少不希望的体重变化。用脲基部分代替乙酰胺部分极大地改善了小鼠ACC1对ACC2的选择性。另外,类似物1b在小鼠盒式给药PK研究中显示出良好的生物利用度,因此也进行了体内PD研究。口服给药1b可以显着降低HCT-116异种移植肿瘤中丙二酰辅酶A的浓度,剂量超过30 mg / kg。此外,化合物1b在786-O异种移植小鼠中口服剂量为30 mg / kg(T / C = 0.5%)时显示出显着的抗肿瘤功效。因此,我们新型的有效ACC1选择性抑制剂代表了一组有用的口服研究工具以及潜在的治疗剂,尤其是在新的癌症治疗方面。


































 京公网安备 11010802027423号
京公网安备 11010802027423号