当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Formal Condensation and [4+1] Annulation Reaction of 3‐Isothiocyanato Oxindoles with Aza‐o‐Quinone Methides
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2019-11-07 , DOI: 10.1002/adsc.201901124 Hou‐Ze Gui 1 , Xiao‐Yun Wu 1 , Yin Wei 2 , Min Shi 1, 2, 3
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2019-11-07 , DOI: 10.1002/adsc.201901124 Hou‐Ze Gui 1 , Xiao‐Yun Wu 1 , Yin Wei 2 , Min Shi 1, 2, 3
Affiliation
|
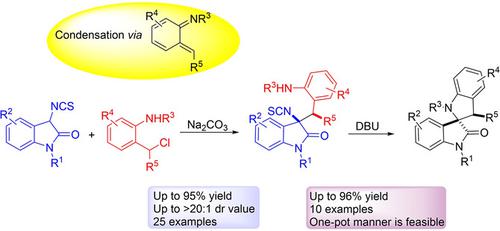
A formal condensation and [4+1] annulation reactions of 3‐isothiocyanato oxindoles with aza‐o‐quinone methides have been reported in this paper for the first time. The use of Na2CO3 as a base exclusively gave the corresponding condensed products in good yields, which can be smoothly transformed into the formal [4+1] annulation products if using DBU as a base. In addition, isothiocyanate could serve as a leaving group during the nucleophilic addition reaction has been disclosed. The two bases play different roles in the reaction and the desired cyclized products can be also obtained in good to high yields in a one‐pot manner under mild conditions.
中文翻译:

3-异硫氰酸根合吲哚与甲基氮杂邻醌的形式缩合和[4 + 1]环化反应
正式缩合和[4 + 1]环反应-3-异硫氰酸酯与羟吲哚氮杂- ö -quinone甲基化物在本文首次被报道。使用Na 2 CO 3作为碱,仅以良好的产率获得了相应的缩合产物,如果使用DBU作为碱,则可以平稳地转化为正式的[4 + 1]环化产物。另外,已经公开了异硫氰酸酯可以在亲核加成反应期间用作离去基团。两种碱在反应中的作用不同,在温和的条件下,也可以一锅法以高收率或高收率获得所需的环化产物。
更新日期:2019-11-07
中文翻译:

3-异硫氰酸根合吲哚与甲基氮杂邻醌的形式缩合和[4 + 1]环化反应
正式缩合和[4 + 1]环反应-3-异硫氰酸酯与羟吲哚氮杂- ö -quinone甲基化物在本文首次被报道。使用Na 2 CO 3作为碱,仅以良好的产率获得了相应的缩合产物,如果使用DBU作为碱,则可以平稳地转化为正式的[4 + 1]环化产物。另外,已经公开了异硫氰酸酯可以在亲核加成反应期间用作离去基团。两种碱在反应中的作用不同,在温和的条件下,也可以一锅法以高收率或高收率获得所需的环化产物。































 京公网安备 11010802027423号
京公网安备 11010802027423号