当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Engineered C-N Lyase: Enantioselective Synthesis of Chiral Synthons for Artificial Dipeptide Sweeteners.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-11-19 , DOI: 10.1002/anie.201910704 Jielin Zhang 1 , Eleonora Grandi 1 , Haigen Fu 1 , Thangavelu Saravanan 1 , Laura Bothof 1 , Pieter G Tepper 1 , Andy-Mark W H Thunnissen 2 , Gerrit J Poelarends 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-11-19 , DOI: 10.1002/anie.201910704 Jielin Zhang 1 , Eleonora Grandi 1 , Haigen Fu 1 , Thangavelu Saravanan 1 , Laura Bothof 1 , Pieter G Tepper 1 , Andy-Mark W H Thunnissen 2 , Gerrit J Poelarends 1
Affiliation
|
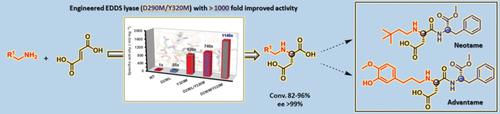
Aspartic acid derivatives with branched N-alkyl or N-arylalkyl substituents are valuable precursors to artificial dipeptide sweeteners such as neotame and advantame. The development of a biocatalyst to synthesize these compounds in a single asymmetric step is an as yet unmet challenge. Reported here is an enantioselective biocatalytic synthesis of various difficult N-substituted aspartic acids, including N-(3,3-dimethylbutyl)-l-aspartic acid and N-[3-(3-hydroxy-4-methoxyphenyl)propyl]-l-aspartic acid, precursors to neotame and advantame, respectively, using an engineered variant of ethylenediamine-N,N'-disuccinic acid (EDDS) lyase from Chelativorans sp. BNC1. This engineered C-N lyase (mutant D290M/Y320M) displayed a remarkable 1140-fold increase in activity for the selective hydroamination of fumarate compared to that of the wild-type enzyme. These results present new opportunities to develop practical multienzymatic processes for the more sustainable and step-economic synthesis of an important class of food additives.
中文翻译:

工程CN裂解酶:用于手性二肽甜味剂的手性合成子的对映选择性合成。
具有支链N-烷基或N-芳基烷基取代基的天冬氨酸衍生物是人造二肽甜味剂例如纽甜和advantame的有价值的前体。在单个不对称步骤中开发合成这些化合物的生物催化剂仍是尚未解决的挑战。在此报道的是对映体选择性生物催化合成的各种困难的N-取代的天冬氨酸,包括N-(3,3-二甲基丁基)-1-天冬氨酸和N- [3-(3-羟基-3-甲氧基苯基)丙基] -1 -天冬氨酸,纽甜和前体的前体,分别使用来自螯虾属的乙二胺-N,N'-二琥珀酸(EDDS)裂解酶的工程变体。BNC1。与野生型酶相比,这种工程改造的CN裂解酶(突变体D290M / Y320M)在富马酸酯的选择性加氢胺化中显示出显着的1140倍活性增加。
更新日期:2019-11-20
中文翻译:

工程CN裂解酶:用于手性二肽甜味剂的手性合成子的对映选择性合成。
具有支链N-烷基或N-芳基烷基取代基的天冬氨酸衍生物是人造二肽甜味剂例如纽甜和advantame的有价值的前体。在单个不对称步骤中开发合成这些化合物的生物催化剂仍是尚未解决的挑战。在此报道的是对映体选择性生物催化合成的各种困难的N-取代的天冬氨酸,包括N-(3,3-二甲基丁基)-1-天冬氨酸和N- [3-(3-羟基-3-甲氧基苯基)丙基] -1 -天冬氨酸,纽甜和前体的前体,分别使用来自螯虾属的乙二胺-N,N'-二琥珀酸(EDDS)裂解酶的工程变体。BNC1。与野生型酶相比,这种工程改造的CN裂解酶(突变体D290M / Y320M)在富马酸酯的选择性加氢胺化中显示出显着的1140倍活性增加。






























 京公网安备 11010802027423号
京公网安备 11010802027423号