当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structurally Modified Cyclopenta[b]benzofuran Analogues Isolated from Aglaia perviridis.
Journal of Natural Products ( IF 3.3 ) Pub Date : 2019-10-17 , DOI: 10.1021/acs.jnatprod.9b00631 Garima Agarwal , James R Wilson , Steven J Kurina 1 , Gerardo D Anaya-Eugenio , Tran N Ninh 2 , Joanna E Burdette 1 , Djaja D Soejarto 2, 3 , Xiaolin Cheng , Esperanza J Carcache de Blanco , L Harinantenaina Rakotondraibe , A Douglas Kinghorn
Journal of Natural Products ( IF 3.3 ) Pub Date : 2019-10-17 , DOI: 10.1021/acs.jnatprod.9b00631 Garima Agarwal , James R Wilson , Steven J Kurina 1 , Gerardo D Anaya-Eugenio , Tran N Ninh 2 , Joanna E Burdette 1 , Djaja D Soejarto 2, 3 , Xiaolin Cheng , Esperanza J Carcache de Blanco , L Harinantenaina Rakotondraibe , A Douglas Kinghorn
Affiliation
|
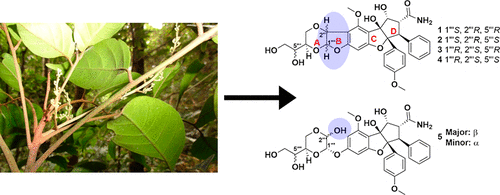
Four new cyclopenta[b]benzofuran derivatives based on an unprecedented carbon skeleton (1-4), with a dihydrofuran ring fused to dioxanyl and aryl rings, along with a new structural analogue (5) of 5‴-episilvestrol (episilvestrol, 7), were isolated from an aqueous extract of a large-scale re-collection of the roots of Aglaia perviridis collected in Vietnam. Compound 5 demonstrated mutarotation in solution due to the presence of a hydroxy group at C-2‴, leading to the isolation of a racemic mixture, despite being purified on a chiral-phase HPLC column. Silvestrol (6) and episilvestrol (7) were isolated from the most potently cytotoxic chloroform subfraction of the roots. All new structures were elucidated using 1D and 2D NMR, HRESIMS, IR, UV, and ECD spectroscopic data. Of the five newly isolated compounds, only compound 5 exhibited cytotoxic activity against a human colon cancer (HT-29) and human prostate cancer cell line (PC-3), with IC50 values of 2.3 μM in both cases. The isolated compounds (1-5) double the number of dioxanyl ring-containing rocaglate analogues reported to date from Aglaia species and present additional information on the structural requirements for cancer cell line cytotoxicity within this compound class.
中文翻译:

从 Aglaia perviridis 中分离出结构修饰的环戊[b]苯并呋喃类似物。
四种新的环戊[b]苯并呋喃衍生物,基于前所未有的碳骨架 (1-4),其中二氢呋喃环与二恶烷基和芳基环稠合,以及 5‴-表西维酚 (episilvestrol, 7) 的新结构类似物 (5) ,是从越南收集的大面积再收集的 Aglaia perviridis 根的水提取物中分离出来的。尽管在手性相 HPLC 柱上进行了纯化,但由于 C-24 处羟基的存在,化合物 5 在溶液中表现出变旋,导致外消旋混合物的分离。 Silvestrol (6) 和 episilvestrol (7) 是从根中细胞毒性最强的氯仿亚组分中分离出来的。所有新结构均使用 1D 和 2D NMR、HRESIMS、IR、UV 和 ECD 光谱数据进行了阐明。在五种新分离的化合物中,只有化合物 5 对人结肠癌 (HT-29) 和人前列腺癌细胞系 (PC-3) 表现出细胞毒活性,两种情况下的 IC50 值为 2.3 μM。分离的化合物 (1-5) 使迄今为止报道的来自 Aglaia 物种的含二恶烷基环的 rocaglate 类似物的数量增加了一倍,并提供了有关此类化合物中癌细胞系细胞毒性的结构要求的附加信息。
更新日期:2019-10-17
中文翻译:

从 Aglaia perviridis 中分离出结构修饰的环戊[b]苯并呋喃类似物。
四种新的环戊[b]苯并呋喃衍生物,基于前所未有的碳骨架 (1-4),其中二氢呋喃环与二恶烷基和芳基环稠合,以及 5‴-表西维酚 (episilvestrol, 7) 的新结构类似物 (5) ,是从越南收集的大面积再收集的 Aglaia perviridis 根的水提取物中分离出来的。尽管在手性相 HPLC 柱上进行了纯化,但由于 C-24 处羟基的存在,化合物 5 在溶液中表现出变旋,导致外消旋混合物的分离。 Silvestrol (6) 和 episilvestrol (7) 是从根中细胞毒性最强的氯仿亚组分中分离出来的。所有新结构均使用 1D 和 2D NMR、HRESIMS、IR、UV 和 ECD 光谱数据进行了阐明。在五种新分离的化合物中,只有化合物 5 对人结肠癌 (HT-29) 和人前列腺癌细胞系 (PC-3) 表现出细胞毒活性,两种情况下的 IC50 值为 2.3 μM。分离的化合物 (1-5) 使迄今为止报道的来自 Aglaia 物种的含二恶烷基环的 rocaglate 类似物的数量增加了一倍,并提供了有关此类化合物中癌细胞系细胞毒性的结构要求的附加信息。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号