当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient Synthesis of α-Allylbutenolides from Allyl Ynoates via Tandem Ligand-Enabled Au(I) Catalysis and the Claisen Rearrangement
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-10-17 , DOI: 10.1021/acscatal.9b03501 Huiyan Wang 1, 2 , Ting Li 1 , Zhitong Zheng 1 , Liming Zhang 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-10-17 , DOI: 10.1021/acscatal.9b03501 Huiyan Wang 1, 2 , Ting Li 1 , Zhitong Zheng 1 , Liming Zhang 1
Affiliation
|
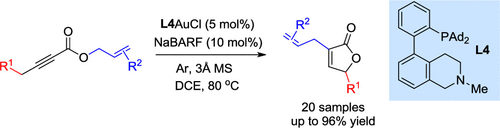
A facile construction of α-allylbutenolides from readily available allyl ynoates has been developed via tandem gold-catalyzed alkyne isomerization to allene and cycloisomerization, the Claisen rearrangement and a double bond migration. The gold catalysis is enabled by a bifunctional phosphine ligand featuring a critical remote tertiary amino group, and the reaction tolerates a range of substituents and exhibits yields up to 96%.
中文翻译:

通过串联配体使能的Au(I)催化和Claisen重排,从壬酸烯丙酯高效合成α-烯丙基丁烯酸内酯
通过串联金催化的炔烃异构化为丙二烯和环异构化,克莱森重排和双键迁移,已经开发了一种由易得的烯丙基壬酸酯容易地构建α-烯丙基丁烯化物的方法。金的催化作用是通过具有关键的远端叔氨基的双功能膦配体实现的,该反应可耐受一系列取代基,收率最高可达96%。
更新日期:2019-10-17
中文翻译:

通过串联配体使能的Au(I)催化和Claisen重排,从壬酸烯丙酯高效合成α-烯丙基丁烯酸内酯
通过串联金催化的炔烃异构化为丙二烯和环异构化,克莱森重排和双键迁移,已经开发了一种由易得的烯丙基壬酸酯容易地构建α-烯丙基丁烯化物的方法。金的催化作用是通过具有关键的远端叔氨基的双功能膦配体实现的,该反应可耐受一系列取代基,收率最高可达96%。































 京公网安备 11010802027423号
京公网安备 11010802027423号