当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rational Design of PLGA Nanoparticle Vaccine Delivery Systems To Improve Immune Responses.
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2019-10-25 , DOI: 10.1021/acs.molpharmaceut.9b00860 Pengfei Gu , Adelijiang Wusiman , Yue Zhang , Zhenguang Liu , Ruonan Bo , Yuanliang Hu , Jiaguo Liu , Deyun Wang
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2019-10-25 , DOI: 10.1021/acs.molpharmaceut.9b00860 Pengfei Gu , Adelijiang Wusiman , Yue Zhang , Zhenguang Liu , Ruonan Bo , Yuanliang Hu , Jiaguo Liu , Deyun Wang
|
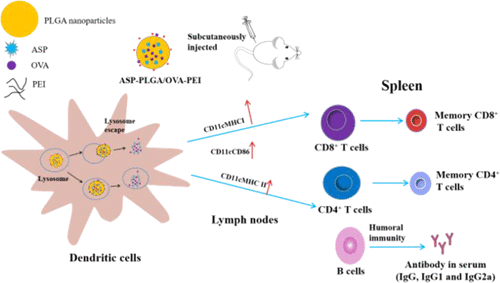
Nanoparticle-based vaccine delivery systems have been extensively used to promote and induce immune responses to protein antigens. The properties of the nanoparticles, such as size, surface charge, and antigen loading mode, have been proved to significantly influence the adjuvant effect and immunoreactivity of nanoparticle-based vaccine delivery systems. The purpose of the study was to investigate how the surface charge and antigen loading mode of nanoparticles impact the immune responses. In this study, three ovalbumin (OVA)-loaded poly(lactic-co-glycolic acid) (PLGA) nanoparticles with different surface charges and antigen loading modes were developed. The three nanoparticles were designed as antigen encapsulated with negatively charged (Angelica sinensis polysaccharide (ASP)-PLGA/OVA), antigen encapsulated with polyethylenimine (PEI)-coated (ASP-PLGA/OVA-PEI), and antigen adsorbed on PEI-coated (ASP-PLGA-PEI-OVA) nanoparticles. The Angelica sinensis polysaccharide (ASP) was used as the immunopotentiator and encapsulated into three nanoparticles. The results demonstrated that both PEI-coated (positively charged) nanoparticles promoted the antigen escape from the endosome, which led to the cytoplasmic antigen delivery to generate cross presentation, compared to negatively charged nanoparticles. In addition, PEI-coated nanoparticles activated the DCs in lymph nodes 5 days after the primary vaccination. In vivo experiments demonstrated that both antigen-encapsulated nanoparticles induced more potent and long-term antigen-specific antibody responses, compared to that of antigen-adsorbed nanoparticles. Thus, the PEI-coated and antigen-encapsulated nanoparticles (ASP-PLGA/OVA-PEI) as a vaccine adjuvant delivery system have the potential to induce strong and long-term humoral and cellular immune responses.
中文翻译:

合理设计PLGA纳米颗粒疫苗输送系统,以改善免疫反应。
基于纳米颗粒的疫苗递送系统已被广泛用于促进和诱导对蛋白质抗原的免疫反应。纳米粒子的性质,例如大小,表面电荷和抗原加载模式,已被证明会显着影响基于纳米粒子的疫苗输送系统的佐剂作用和免疫反应性。该研究的目的是研究纳米粒子的表面电荷和抗原加载方式如何影响免疫反应。在这项研究中,三个卵清蛋白(OVA)-loaded聚(乳酸-共开发了具有不同表面电荷和抗原加载方式的β-乙醇酸(PLGA)纳米粒子。将这三个纳米颗粒设计为用带负电荷的包被抗原(当归多糖(ASP)-PLGA / OVA),用聚乙烯亚胺(PEI)包被的抗原(ASP-PLGA / OVA-PEI)包被的抗原以及吸附在PEI包被的抗原(ASP-PLGA-PEI-OVA)纳米粒子。当归多糖(ASP)用作免疫增强剂,并封装成三个纳米粒子。结果表明,与带负电的纳米颗粒相比,两种PEI包覆的(带正电荷的)纳米颗粒均能促进抗原从内体中逸出,从而导致细胞质抗原递送产生交叉呈递。此外,初次接种疫苗5天后,涂有PEI的纳米颗粒激活了淋巴结中的DC。体内实验表明,与抗原吸附的纳米颗粒相比,抗原包裹的纳米颗粒均诱导更有效和长期的抗原特异性抗体反应。因此,作为疫苗佐剂递送系统的PEI包被的和抗原包封的纳米颗粒(ASP-PLGA / OVA-PEI)具有诱导强而长期的体液和细胞免疫应答的潜力。
更新日期:2019-10-25
中文翻译:

合理设计PLGA纳米颗粒疫苗输送系统,以改善免疫反应。
基于纳米颗粒的疫苗递送系统已被广泛用于促进和诱导对蛋白质抗原的免疫反应。纳米粒子的性质,例如大小,表面电荷和抗原加载模式,已被证明会显着影响基于纳米粒子的疫苗输送系统的佐剂作用和免疫反应性。该研究的目的是研究纳米粒子的表面电荷和抗原加载方式如何影响免疫反应。在这项研究中,三个卵清蛋白(OVA)-loaded聚(乳酸-共开发了具有不同表面电荷和抗原加载方式的β-乙醇酸(PLGA)纳米粒子。将这三个纳米颗粒设计为用带负电荷的包被抗原(当归多糖(ASP)-PLGA / OVA),用聚乙烯亚胺(PEI)包被的抗原(ASP-PLGA / OVA-PEI)包被的抗原以及吸附在PEI包被的抗原(ASP-PLGA-PEI-OVA)纳米粒子。当归多糖(ASP)用作免疫增强剂,并封装成三个纳米粒子。结果表明,与带负电的纳米颗粒相比,两种PEI包覆的(带正电荷的)纳米颗粒均能促进抗原从内体中逸出,从而导致细胞质抗原递送产生交叉呈递。此外,初次接种疫苗5天后,涂有PEI的纳米颗粒激活了淋巴结中的DC。体内实验表明,与抗原吸附的纳米颗粒相比,抗原包裹的纳米颗粒均诱导更有效和长期的抗原特异性抗体反应。因此,作为疫苗佐剂递送系统的PEI包被的和抗原包封的纳米颗粒(ASP-PLGA / OVA-PEI)具有诱导强而长期的体液和细胞免疫应答的潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号