当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Predicting an Antiaromatic Benzene Ring in the Ground State Caused by Hyperconjugation.
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2019-11-06 , DOI: 10.1002/asia.201901261 Yu Zhao 1 , Qiong Xie 1 , Tingting Sun 1 , Jiashun Wu 1 , Jun Zhu 1
Chemistry - An Asian Journal ( IF 3.5 ) Pub Date : 2019-11-06 , DOI: 10.1002/asia.201901261 Yu Zhao 1 , Qiong Xie 1 , Tingting Sun 1 , Jiashun Wu 1 , Jun Zhu 1
Affiliation
|
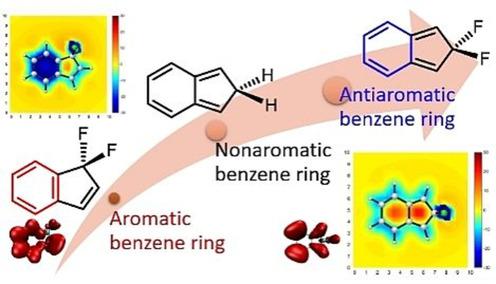
Benzene, the prototype of aromatics, has six equivalent C-C bonds (1.397 Å), which are intermediate between a C-C double bond and a C-C single bond. For over 80 years, chemists have spent much effort on freezing a localized structure to obtain a distorted bond-length alternating benzene ring in the ground state, leading to various localized trisannelated benzene rings. However, most of the central benzene rings are still aromatic or nonaromatic. Here we report an antiaromatic benzene ring caused by hyperconjugation. Specifically, symmetric annulation of 5,5-difluorocyclopentadiene results in an antiaromatic benzene ring, which is supported by various aromaticity indices, including nucleus-independent chemical shift, anisotropy of the induced current density, π-separated electron-localization function and heat of hydrogenation. Our findings highlight a strong power of hyperconjugation, a "weak" interaction in organic chemistry, paving the way for designing and realizing more novel (anti)aromatics.
中文翻译:

预测由超共轭作用引起的基态抗芳烃苯环。
苯是芳烃的原型,具有六个等效的CC键(1.397Å),位于CC双键和CC单键之间。80多年来,化学家们花了很大的精力来冻结局部结构,以获得基态扭曲的键长交替的苯环,从而导致出现各种局部的三锡烷基苯环。但是,大多数中心苯环仍是芳族或非芳族的。在这里,我们报告了由超共轭作用引起的抗芳香族苯环。具体而言,对称的5,5-二氟环戊二烯环化会生成一个抗芳族苯环,该环由各种芳香指数支持,包括与核无关的化学位移,感应电流密度的各向异性,π分离的电子定位功能和氢化热。
更新日期:2019-11-06
中文翻译:

预测由超共轭作用引起的基态抗芳烃苯环。
苯是芳烃的原型,具有六个等效的CC键(1.397Å),位于CC双键和CC单键之间。80多年来,化学家们花了很大的精力来冻结局部结构,以获得基态扭曲的键长交替的苯环,从而导致出现各种局部的三锡烷基苯环。但是,大多数中心苯环仍是芳族或非芳族的。在这里,我们报告了由超共轭作用引起的抗芳香族苯环。具体而言,对称的5,5-二氟环戊二烯环化会生成一个抗芳族苯环,该环由各种芳香指数支持,包括与核无关的化学位移,感应电流密度的各向异性,π分离的电子定位功能和氢化热。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号