当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxygen Vacancy-rich Porous Co3O4 Nanosheets toward Boosted NO Reduction by CO and CO Oxidation: Insights into the Structure–Activity Relationship and Performance Enhancement Mechanism
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2019-10-30 , DOI: 10.1021/acsami.9b08664 Xinyang Wang 1 , Xinyong Li 1, 2 , Jincheng Mu 1 , Shiying Fan 1 , Xin Chen 1 , Liang Wang 1 , Zhifan Yin 1 , Moses Tadé 2 , Shaomin Liu 2
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2019-10-30 , DOI: 10.1021/acsami.9b08664 Xinyang Wang 1 , Xinyong Li 1, 2 , Jincheng Mu 1 , Shiying Fan 1 , Xin Chen 1 , Liang Wang 1 , Zhifan Yin 1 , Moses Tadé 2 , Shaomin Liu 2
Affiliation
|
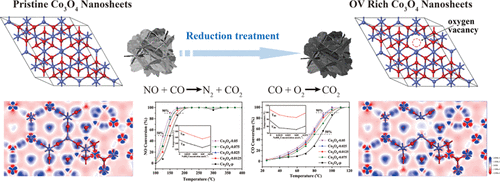
Oxygen vacancy-rich porous Co3O4 nanosheets (OV-Co3O4) with diverse surface oxygen vacancy contents were synthesized via facile surface reduction and applied to NO reduction by CO and CO oxidation. The structure–activity relationship between surface oxygen vacancies and catalytic performance was systematically investigated. By combining Raman, X-ray diffraction, transmission electron microscopy, X-ray photoelectron spectroscopy, and O2-temperature programmed desorption, it was found that the efficient surface reduction leads to the presence of more surface oxygen vacancies and thus distinctly enhance the surface oxygen amount and mobility of OV-Co3O4. The electron transfer towards Co sites was promoted by surface oxygen vacancies with higher content. Compared with the pristine porous Co3O4 nanosheets, the presence of more surface oxygen vacancies is beneficial for the catalytic performance enhancement for NO reduction by CO and CO oxidation. The OV-Co3O4 obtained in 0.05 mol L–1 NaBH4 solution (Co3O4-0.05) exhibited the best catalytic activity, achieving 100% NO conversion at 175 °C in NO reduction by CO and 100% CO conversion at 100 °C in CO oxidation, respectively. Co3O4-0.05 exhibited outstanding catalytic stability and resistance to high gas hour space velocity in both reactions. Combining in situ DRIFTS results, the enhanced performance of OV-Co3O4 for NO reduction by CO should be attributed to the promoted formation and transformation of dinitrosyl species and −NCO species at lower and higher temperatures. The enhanced performance of OV-Co3O4 for CO oxidation is due to the promotion of oxygen activation ability, surface oxygen mobility, as well as the enhanced CO2 desorption ability. The results indicate that the direct regulation of surface oxygen vacancies could be an efficient way to evidently enhance the catalytic performance for NO reduction by CO and CO oxidation.
中文翻译:

富氧空缺的多孔Co 3 O 4纳米片,通过CO和CO氧化促进NO还原:洞察结构-活性关系和性能增强机制
通过容易的表面还原,合成了具有多种表面氧空位含量的富氧空位的多孔Co 3 O 4纳米片(OV-Co 3 O 4),并将其应用于通过CO和CO氧化进行NO还原。系统地研究了表面氧空位与催化性能之间的构效关系。通过结合拉曼光谱,X射线衍射,透射电子显微镜,X射线光电子能谱和O 2温度程序解吸,发现有效的表面还原导致存在更多的表面氧空位,从而显着增强表面氧量和OV-Co 3 O 4的迁移率。具有较高含量的表面氧空位促进了电子向Co位的转移。与原始多孔的Co 3 O 4纳米片相比,存在更多的表面氧空位有利于增强通过CO和CO氧化还原NO的催化性能。在0.05 mol L –1 NaBH 4溶液(Co 3 O 4 -0.05)中获得的OV-Co 3 O 4表现出最佳的催化活性,在175°C时实现了100%的NO转化率,而通过CO还原NO和100%的CO转化率分别在100°C的CO中氧化。钴3 O 4-0.05在两个反应中均表现出出色的催化稳定性和对高气体时空速度的抵抗力。结合原位DRIFTS结果,OV-Co 3 O 4通过CO还原NO的性能增强应归因于在较低和较高的温度下促进了二亚硝酰基物质和-NCO物质的形成和转化。OV-Co 3 O 4用于CO氧化的性能增强是由于提高了氧活化能力,表面氧迁移率以及增强了的CO 2解吸能力。结果表明,直接调节表面氧空位可能是一种有效的方法,可以明显提高CO和CO氧化还原NO的催化性能。
更新日期:2019-10-30
中文翻译:

富氧空缺的多孔Co 3 O 4纳米片,通过CO和CO氧化促进NO还原:洞察结构-活性关系和性能增强机制
通过容易的表面还原,合成了具有多种表面氧空位含量的富氧空位的多孔Co 3 O 4纳米片(OV-Co 3 O 4),并将其应用于通过CO和CO氧化进行NO还原。系统地研究了表面氧空位与催化性能之间的构效关系。通过结合拉曼光谱,X射线衍射,透射电子显微镜,X射线光电子能谱和O 2温度程序解吸,发现有效的表面还原导致存在更多的表面氧空位,从而显着增强表面氧量和OV-Co 3 O 4的迁移率。具有较高含量的表面氧空位促进了电子向Co位的转移。与原始多孔的Co 3 O 4纳米片相比,存在更多的表面氧空位有利于增强通过CO和CO氧化还原NO的催化性能。在0.05 mol L –1 NaBH 4溶液(Co 3 O 4 -0.05)中获得的OV-Co 3 O 4表现出最佳的催化活性,在175°C时实现了100%的NO转化率,而通过CO还原NO和100%的CO转化率分别在100°C的CO中氧化。钴3 O 4-0.05在两个反应中均表现出出色的催化稳定性和对高气体时空速度的抵抗力。结合原位DRIFTS结果,OV-Co 3 O 4通过CO还原NO的性能增强应归因于在较低和较高的温度下促进了二亚硝酰基物质和-NCO物质的形成和转化。OV-Co 3 O 4用于CO氧化的性能增强是由于提高了氧活化能力,表面氧迁移率以及增强了的CO 2解吸能力。结果表明,直接调节表面氧空位可能是一种有效的方法,可以明显提高CO和CO氧化还原NO的催化性能。






























 京公网安备 11010802027423号
京公网安备 11010802027423号