当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interface Engineering of an RGO/MoS2/Pd 2D Heterostructure for Electrocatalytic Overall Water Splitting in Alkaline Medium
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2019-10-29 , DOI: 10.1021/acsami.9b13358 Ayushi Pandey 1 , Ayan Mukherjee 1 , Sankalpita Chakrabarty 1 , Debabrata Chanda 1 , Suddhasatwa Basu 1, 2
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2019-10-29 , DOI: 10.1021/acsami.9b13358 Ayushi Pandey 1 , Ayan Mukherjee 1 , Sankalpita Chakrabarty 1 , Debabrata Chanda 1 , Suddhasatwa Basu 1, 2
Affiliation
|
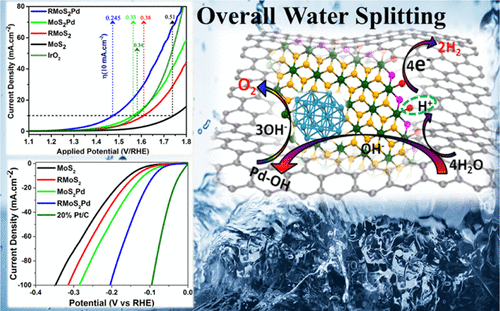
To achieve sustainable production of H2 at ambient temperature, highly active and stable electrocatalysts are the key to water splitting technology commercialization for hydrogen and oxygen production to replace Pt and IrO2 catalysts. Herein, a modified interface of palladium (Pd) and reduced graphene oxide (RGO)-supported molybdenum disulfide (MoS2) prepared by the solvothermal followed by chemical reduction method is established, in which abundant interfaces are formed. The phase structure, composition, chemical coupling, and morphology of the two-dimensional nanostructures are established by X-ray diffraction (XRD), Raman spectroscopy, X-ray photoelectron spectroscopy (XPS), and transmission electron microscopy, respectively. A structural phase transformation in MoS2 is observed from trigonal (2H) to octahedral (1T) by virtue of Pd addition, which is well established from XRD, Raman, and XPS studies. For oxygen evolution reaction (OER) and hydrogen evolution reaction (HER), the RGO/MoS2/Pd (RMoS2Pd) catalyst exhibits extremely low overpotential (245 mV for OER and 86 mV for HER) to achieve benchmark current density, with small values of Tafel slope (42 mV dec–1 for OER and 35.9 mV dec–1 for HER) and charge transfer resistance. The quantitative study shows the hydrogen production rate of RMoS2Pd of 335 μmol h–1 with excellent stability in alkaline medium, which is superior to MoS2, RMoS2, and MoS2Pd. The improved performance of RMoS2Pd is attributed to the combined synergetic effect of 1T MoS2, sulfur vacancy, and conducting RGO sheet, which efficiently accelerate the overall electrochemical water splitting.
中文翻译:

RGO / MoS 2 / Pd 2D异质结构在碱性介质中电催化总水分解的界面工程
为了在环境温度下实现H 2的可持续生产,高活性和稳定的电催化剂是水分解技术商业化生产氢气和氧气以替代Pt和IrO 2催化剂的关键。本文中,钯(Pd)和还原型氧化石墨烯(RGO)负载的二硫化钼(MoS 2建立了由溶剂热然后化学还原的方法制备的),在其中形成了丰富的界面。分别通过X射线衍射(XRD),拉曼光谱,X射线光电子能谱(XPS)和透射电子显微镜来建立二维纳米结构的相结构,组成,化学偶联和形态。通过X射线衍射,拉曼和XPS研究已经很好地确定了Pd的添加,观察到MoS 2中的结构相变从三角形(2H)转变为八面体(1T)。对于氧气析出反应(OER)和氢气析出反应(HER),RGO / MoS 2 / Pd(RMoS 2Pd)的催化剂表现出非常低的超电势(245毫伏OER和86毫伏HER),以实现基准电流密度,与塔菲尔斜率(的小值42毫伏癸-1为OER和35.9毫伏癸-1为HER)和电荷转移反抗。定量研究表明,RMoS 2 Pd的制氢速率为335μmolh -1,在碱性介质中具有出色的稳定性,优于MoS 2,RMoS 2和MoS 2 Pd。RMoS 2 Pd性能的提高归因于1T MoS 2的协同协同作用,硫空位和传导RGO薄板,可有效加速整体电化学水分解。
更新日期:2019-10-29
中文翻译:

RGO / MoS 2 / Pd 2D异质结构在碱性介质中电催化总水分解的界面工程
为了在环境温度下实现H 2的可持续生产,高活性和稳定的电催化剂是水分解技术商业化生产氢气和氧气以替代Pt和IrO 2催化剂的关键。本文中,钯(Pd)和还原型氧化石墨烯(RGO)负载的二硫化钼(MoS 2建立了由溶剂热然后化学还原的方法制备的),在其中形成了丰富的界面。分别通过X射线衍射(XRD),拉曼光谱,X射线光电子能谱(XPS)和透射电子显微镜来建立二维纳米结构的相结构,组成,化学偶联和形态。通过X射线衍射,拉曼和XPS研究已经很好地确定了Pd的添加,观察到MoS 2中的结构相变从三角形(2H)转变为八面体(1T)。对于氧气析出反应(OER)和氢气析出反应(HER),RGO / MoS 2 / Pd(RMoS 2Pd)的催化剂表现出非常低的超电势(245毫伏OER和86毫伏HER),以实现基准电流密度,与塔菲尔斜率(的小值42毫伏癸-1为OER和35.9毫伏癸-1为HER)和电荷转移反抗。定量研究表明,RMoS 2 Pd的制氢速率为335μmolh -1,在碱性介质中具有出色的稳定性,优于MoS 2,RMoS 2和MoS 2 Pd。RMoS 2 Pd性能的提高归因于1T MoS 2的协同协同作用,硫空位和传导RGO薄板,可有效加速整体电化学水分解。































 京公网安备 11010802027423号
京公网安备 11010802027423号