当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reconsidering Calcium Dehydration as the Rate-Determining Step in Calcium Mineral Growth
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-10-29 , DOI: 10.1021/acs.jpcc.9b06403 Janou A Koskamp 1 , Sergio E Ruiz-Hernandez 1 , Devis Di Tommaso 2 , Alin Marin Elena 3 , Nora H De Leeuw 1, 4 , Mariette Wolthers 1
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-10-29 , DOI: 10.1021/acs.jpcc.9b06403 Janou A Koskamp 1 , Sergio E Ruiz-Hernandez 1 , Devis Di Tommaso 2 , Alin Marin Elena 3 , Nora H De Leeuw 1, 4 , Mariette Wolthers 1
Affiliation
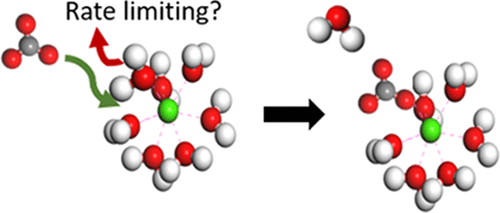
|
The dehydration of cations is generally accepted as the rate-limiting step in many processes. Molecular dynamics (MD) can be used to investigate the dynamics of water molecules around cations, and two different methods exist to obtain trajectory-based water dehydration frequencies. Here, these two different post-processing methods (direct method versus survival function) have been implemented to obtain calcium dehydration frequencies from a series of trajectories obtained using a range of accepted force fields. None of the method combinations reproduced the commonly accepted experimental water exchange frequency of 10–8.2 s–1. Instead, our results suggest much faster water dynamics, comparable with more accurate ab initio MD simulations and with experimental values obtained using neutron scattering techniques. We obtained the best agreement using the survival function method to characterize the water dynamics, and we show that different method combinations significantly affect the outcome. Our work strongly suggests that the fast water exchange kinetics around the calcium ions is not rate-limiting for reactions involving dissolved/solvated calcium. Our results further suggest that, for alkali and most of the earth alkali metals, mechanistic rate laws for growth, dissolution, and adsorption, which are based on the principle of rate-limiting cation dehydration, need careful reconsideration.
中文翻译:

重新考虑钙脱水作为钙矿物质生长的速率决定步骤
阳离子脱水通常被认为是许多过程中的限速步骤。分子动力学(MD)可用于研究阳离子周围水分子的动力学,并且存在两种不同的方法来获得基于轨迹的水脱水频率。在这里,这两种不同的后处理方法(直接方法与生存函数)已被实施,以从使用一系列可接受的力场获得的一系列轨迹中获得钙脱水频率。没有一种方法组合能够重现普遍接受的实验水交换频率 10 –8.2 s –1。相反,我们的结果表明水动力学要快得多,与更准确的从头算MD模拟和使用中子散射技术获得的实验值相当。我们使用生存函数方法来表征水动力学获得了最佳一致性,并且我们表明不同的方法组合会显着影响结果。我们的工作强烈表明,钙离子周围的快速水交换动力学并不是涉及溶解/溶剂化钙的反应的速率限制。我们的结果进一步表明,对于碱金属和大多数碱土金属,基于限速阳离子脱水原理的生长、溶解和吸附的机械速率定律需要仔细重新考虑。
更新日期:2019-10-29
中文翻译:

重新考虑钙脱水作为钙矿物质生长的速率决定步骤
阳离子脱水通常被认为是许多过程中的限速步骤。分子动力学(MD)可用于研究阳离子周围水分子的动力学,并且存在两种不同的方法来获得基于轨迹的水脱水频率。在这里,这两种不同的后处理方法(直接方法与生存函数)已被实施,以从使用一系列可接受的力场获得的一系列轨迹中获得钙脱水频率。没有一种方法组合能够重现普遍接受的实验水交换频率 10 –8.2 s –1。相反,我们的结果表明水动力学要快得多,与更准确的从头算MD模拟和使用中子散射技术获得的实验值相当。我们使用生存函数方法来表征水动力学获得了最佳一致性,并且我们表明不同的方法组合会显着影响结果。我们的工作强烈表明,钙离子周围的快速水交换动力学并不是涉及溶解/溶剂化钙的反应的速率限制。我们的结果进一步表明,对于碱金属和大多数碱土金属,基于限速阳离子脱水原理的生长、溶解和吸附的机械速率定律需要仔细重新考虑。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号