Phytomedicine ( IF 6.7 ) Pub Date : 2018-03-06 , DOI: 10.1016/j.phymed.2018.03.007 Mengze Zhou 1 , Suning Li 2 , Ling Song 1 , Qinghua Hu 3 , Wentao Liu 1
|
Backgound
Gout is an inflammatory arthritis characterized by abrupt self-limiting attacks of inflammation caused by precipitation of monosodium urate crystals (MSU) in the joint. Both anti-hyperuricemia and anti-inflammation could be gout therapeutic strategies, whereas ideal drugs for gout treatment are deficient.
Purpose
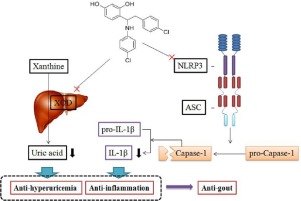
4-(2-(4-chlorophenyl)-1-((4-chlorophenyl)amino)ethyl)benzene-1, 3-diol (CBED) was obtained from a cluster of deoxybenzoins derivatives synthesized by our research group with potent anti-hyperuricemic and anti-inflammatory activities, which was expected to be a dual inhibitor of xanthine oxidase (XOD) and NOD-like receptor protein 3 (NLRP3). This study aimed to investigate effects of CBED on XOD and NLRP3 in vitro, as well as the possible mechanisms by which CBED improved gout in vivo.
Methods
After molecular docking detection, inhibitory effects of CBED on XOD and NLRP3 were evaluated in vitro. Subsequently, hyperuricemia and acute gouty arthritis animal models were established by potassium oxonate or MSU, respectively. After CBED treatment, serum uric acid levels, synovial interleukin (IL)-1β concentrations, hepatic XOD activities, as well as synovial morphological changes were examined. More importantly, synovial expressions of NLRP3 inflammasome components including NLRP3, apoptosis-associated speck-like protein (ASC) and caspase-1 in rats were analyzed by immunofluorescence and western blot.
Results
In vitro, CBED obviously inhibited XOD activity with an IC50 value of 3.87 µM, moreover, it effectively inhibited MSU-induced NLRP3 inflammasome activation and IL-1β over-production in THP-1 cells. In addition, CBED dose-dependently decreased serum uric acid levels suppressed hepatic XOD activities in oxonate-induced hyperuricemic mice. On the other hand, CBED significantly improved MSU-induced ankle swelling and histopathological damage with elevated IL-1β. In addition, NLRP3 inflammasome activation could be blocked by CBED treatment in rats with acute gouty arthritis. Notbly, CBED exhibited no effects on all these indicators in normal animals, predicting its safety.
Conclusions
CBED might serve as a dual XOD and NLRP3 inhibitor for treatment of gout.
中文翻译:

4-(2-(4-chlorophenyl)-1-((4-chlorophenyl)amino)ethyl)benzo-1, 3-diol 是一种潜在的痛风治疗药物,可作为 XOD 和 NLRP3 的双重抑制剂
背景
痛风是一种炎症性关节炎,其特征是由关节中尿酸单钠晶体 (MSU) 沉淀引起的炎症突然自限性发作。抗高尿酸血症和抗炎都可以作为痛风的治疗策略,而治疗痛风的理想药物是缺乏的。
目的
4-(2-(4-chlorophenyl)-1-((4-chlorophenyl)amino)ethyl)benzo-1, 3-diol (CBED) 是从我们课题组合成的具有强抗高尿酸血症和抗炎活性,预计是黄嘌呤氧化酶 (XOD) 和 NOD 样受体蛋白 3 (NLRP3) 的双重抑制剂。本研究旨在研究 CBED 在体外对 XOD 和 NLRP3 的影响,以及 CBED 在体内改善痛风的可能机制。
方法
分子对接检测后,体外评价CBED对XOD和NLRP3的抑制作用。随后,分别用氧酸钾或MSU建立了高尿酸血症和急性痛风性关节炎动物模型。CBED治疗后,检查血清尿酸水平、滑膜白细胞介素(IL)-1β浓度、肝XOD活性以及滑膜形态变化。更重要的是,通过免疫荧光和蛋白质印迹分析了大鼠滑膜中 NLRP3 炎性体成分的表达,包括 NLRP3、凋亡相关斑点样蛋白 (ASC) 和 caspase-1。
结果
在体外,CBED明显抑制XOD活性,IC 50值为3.87 µM,而且它有效抑制MSU诱导的THP-1细胞中NLRP3炎性体活化和IL-1β过度产生。此外,CBED 剂量依赖性地降低血清尿酸水平可抑制含氧酸盐诱导的高尿酸血症小鼠的肝脏 XOD 活性。另一方面,CBED 通过升高的 IL-1β 显着改善 MSU 引起的踝关节肿胀和组织病理学损伤。此外,在患有急性痛风性关节炎的大鼠中,CBED 治疗可以阻断 NLRP3 炎症小体的激活。值得注意的是,CBED 对正常动物的所有这些指标都没有影响,这预示着它的安全性。
结论
CBED 可作为治疗痛风的双重 XOD 和 NLRP3 抑制剂。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号