当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CyClick Chemistry for the Synthesis of Cyclic Peptides.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-11-07 , DOI: 10.1002/anie.201911900 Victor Adebomi 1 , Ryan D Cohen 2, 3 , Rachel Wills 1 , Holland Andrew Hays Chavers 1 , Gary E Martin 2, 3 , Monika Raj 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-11-07 , DOI: 10.1002/anie.201911900 Victor Adebomi 1 , Ryan D Cohen 2, 3 , Rachel Wills 1 , Holland Andrew Hays Chavers 1 , Gary E Martin 2, 3 , Monika Raj 1
Affiliation
|
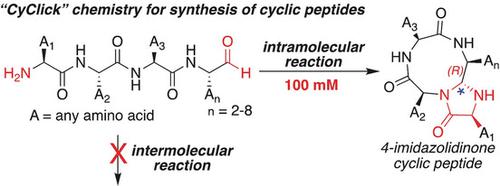
Here, we report a novel "CyClick" strategy for the macrocyclization of peptides that works in an exclusively intramolecular fashion thereby precluding the formation of dimers and oligomers via intermolecular reactions. The CyClick chemistry is highly chemoselective for the N-terminus of the peptide with a C-terminal aldehyde. In this protocol, the peptide conformation internally directs activation of the backbone amide bond and thereby facilitates formation of a stable 4-imidazolidinone-fused cyclic peptide with high diastereoselectivity (>99 %). This method is tolerant to a variety of peptide aldehydes and has been applied for the synthesis of 12- to 23-membered rings with varying amino acid compositions in one pot under mild reaction conditions. The reaction generated peptide macrocycles featuring a 4-imidazolidinone in their scaffolds, which acts as an endocyclic control element that promotes intramolecular hydrogen bonding and leads to macrocycles with conformationally rigid turn structures.
中文翻译:

环肽合成的CyClick化学。
在这里,我们报告了一种新颖的“ CyClick”策略,可对肽进行大分子环化,该策略仅以分子内方式起作用,从而排除了通过分子间反应形成二聚体和寡聚体的可能性。CyClick化学对具有C末端醛基的肽的N末端具有高度的化学选择性。在该方案中,肽构象在内部指导骨架酰胺键的活化,从而促进形成具有高非对映选择性(> 99%)的稳定的4-咪唑啉酮-融合的环状肽。该方法可耐受多种肽醛,并已被用于在温和的反应条件下在一罐中合成具有不同氨基酸组成的12至23元环。
更新日期:2019-11-07
中文翻译:

环肽合成的CyClick化学。
在这里,我们报告了一种新颖的“ CyClick”策略,可对肽进行大分子环化,该策略仅以分子内方式起作用,从而排除了通过分子间反应形成二聚体和寡聚体的可能性。CyClick化学对具有C末端醛基的肽的N末端具有高度的化学选择性。在该方案中,肽构象在内部指导骨架酰胺键的活化,从而促进形成具有高非对映选择性(> 99%)的稳定的4-咪唑啉酮-融合的环状肽。该方法可耐受多种肽醛,并已被用于在温和的反应条件下在一罐中合成具有不同氨基酸组成的12至23元环。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号