当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Properties of the Interphase Formed between Argyrodite-Type Li6PS5Cl and Polymer-Based PEO10:LiTFSI
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2019-10-31 , DOI: 10.1021/acsami.9b14506
Fabian J. Simon 1, 2 , Matthias Hanauer 1 , Anja Henss 2, 3 , Felix H. Richter 2, 3 , Jürgen Janek 2, 3
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2019-10-31 , DOI: 10.1021/acsami.9b14506
Fabian J. Simon 1, 2 , Matthias Hanauer 1 , Anja Henss 2, 3 , Felix H. Richter 2, 3 , Jürgen Janek 2, 3
Affiliation
|
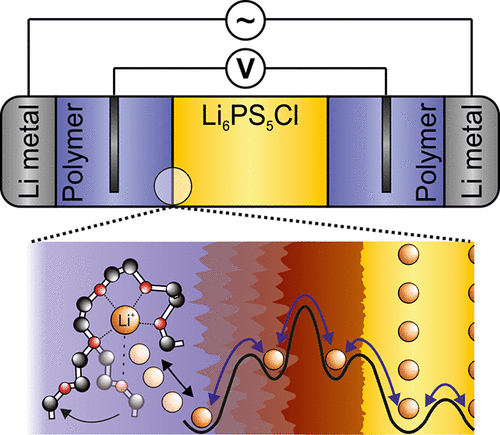
All-solid-state lithium metal batteries using thiophosphate solid electrolytes (SE) present a promising alternative to state-of-the-art lithium-ion batteries due to their potentially superior energy and power. However, reactions occurring at the lithium metal | SE interface result in an increasing internal resistance and limited cycle life. A stable solid polymer electrolyte (SPE) may be used as protective interlayer to prevent the SE from direct contact and reaction with lithium metal. This creates a new and rarely studied heteroionic interface between the inorganic SE and the SPE, which we investigate here. The interface resistance between argyrodite-type Li6PS5Cl and a poly(ethylene oxide)/LiTFSI-based SPE is quantified by four-point electrochemical impedance measurements using two wire-shaped reference electrodes (2.4 Ω cm2 at 80 °C). Two distinct processes are observed and attributed to lithium-ion conduction through a formed solid-polymer electrolyte interphase (SPEI) and an ionic charge-transfer (CT) process. The SPEI predominantly consists of polysulfides and lithium fluoride (LiF), as identified by X-ray photoelectron spectroscopy (XPS) analysis. A temperature-enhanced SPEI growth is observed using electrochemical impedance spectroscopy (EIS) and depth profiling combined with time-of-flight secondary ion mass spectrometry (ToF-SIMS). The results highlight the importance of four-point measurements to determine electrolyte-electrolyte interface properties. Overall, the low resistance and low activation energy of the SPEI makes the SPE interlayer an attractive candidate to protect Li6PS5Cl from decomposition at the lithium metal anode.
中文翻译:

菱铁矿型Li 6 PS 5 Cl与聚合物基PEO 10:LiTFSI之间形成的相变性质
由于使用了硫代磷酸盐固体电解质(SE)的全固态锂金属电池具有潜在的优越能量和功率,因此可以替代最先进的锂离子电池。然而,在锂金属上发生的反应 SE接口导致内部电阻增加,循环寿命有限。稳定的固态聚合物电解质(SPE)可用作保护性中间层,以防止SE与锂金属直接接触并发生反应。这在无机SE和SPE之间创建了一个新的且很少研究的杂离子界面,我们将在此进行研究。菱锰矿型Li 6 PS 5之间的界面电阻Cl和基于聚环氧乙烷/ LiTFSI的SPE通过使用两个线形参比电极(2.4Ωcm 2在80°C时)。观察到两个不同的过程,这归因于通过形成的固体-聚合物电解质中间相(SPEI)和离子电荷转移(CT)过程进行的锂离子传导。通过X射线光电子能谱(XPS)分析确定,SPEI主要由多硫化物和氟化锂(LiF)组成。使用电化学阻抗谱(EIS)和深度分析结合飞行时间二次离子质谱(ToF-SIMS),观察到温度增强的SPEI的生长。结果强调了四点测量对确定电解质-电解质界面特性的重要性。总体而言,SPEI的低电阻和低活化能使SPE中间层成为保护Li 6 PS 5的有吸引力的候选材料Cl从锂金属阳极分解而来。
更新日期:2019-11-01
中文翻译:

菱铁矿型Li 6 PS 5 Cl与聚合物基PEO 10:LiTFSI之间形成的相变性质
由于使用了硫代磷酸盐固体电解质(SE)的全固态锂金属电池具有潜在的优越能量和功率,因此可以替代最先进的锂离子电池。然而,在锂金属上发生的反应 SE接口导致内部电阻增加,循环寿命有限。稳定的固态聚合物电解质(SPE)可用作保护性中间层,以防止SE与锂金属直接接触并发生反应。这在无机SE和SPE之间创建了一个新的且很少研究的杂离子界面,我们将在此进行研究。菱锰矿型Li 6 PS 5之间的界面电阻Cl和基于聚环氧乙烷/ LiTFSI的SPE通过使用两个线形参比电极(2.4Ωcm 2在80°C时)。观察到两个不同的过程,这归因于通过形成的固体-聚合物电解质中间相(SPEI)和离子电荷转移(CT)过程进行的锂离子传导。通过X射线光电子能谱(XPS)分析确定,SPEI主要由多硫化物和氟化锂(LiF)组成。使用电化学阻抗谱(EIS)和深度分析结合飞行时间二次离子质谱(ToF-SIMS),观察到温度增强的SPEI的生长。结果强调了四点测量对确定电解质-电解质界面特性的重要性。总体而言,SPEI的低电阻和低活化能使SPE中间层成为保护Li 6 PS 5的有吸引力的候选材料Cl从锂金属阳极分解而来。







































 京公网安备 11010802027423号
京公网安备 11010802027423号