当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure-Dependent Modulation of Substrate Binding and Biodegradation Activity of Pirin Proteins toward Plant Flavonols.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2019-10-23 , DOI: 10.1021/acschembio.9b00575 Bin Guo 1 , Yichen Zhang 2 , Gregory Hicks 2 , Xingrong Huang 1 , Rongfeng Li 3 , Natalie Roy 2 , Zongchao Jia 2
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2019-10-23 , DOI: 10.1021/acschembio.9b00575 Bin Guo 1 , Yichen Zhang 2 , Gregory Hicks 2 , Xingrong Huang 1 , Rongfeng Li 3 , Natalie Roy 2 , Zongchao Jia 2
Affiliation
|
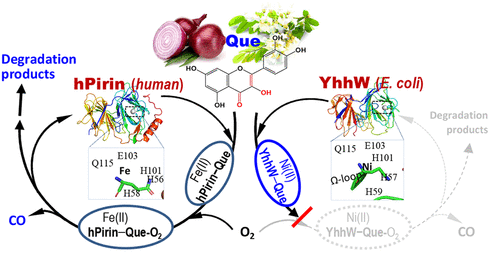
Pirin is a nonheme metalloprotein that occurs widely in human tissues and is highly conserved across all taxa. Pirin proteins typically function as nuclear transcription regulators, but two Pirin orthologs, YhhW (from Escherichia coli) and hPirin (from humans) were revealed to possess enzymatic activity of degrading quercetin. The exact role of Pirin homologues and their catalytic specificity remain poorly understood. In this work, by screening against a panel of plant flavonoids, we found that both Pirins catalyze the oxidative degradation of a wide spectrum of flavonol analogues and release carbon monoxide (CO) in the process. This shows that Pirin acts on a broad range of substrates and could represent a novel dietary source of CO in vivo. Although the kinetic profiles differ substantially between two Pirins, the identified substrate structures all share a 2,3-double bond and 3-hydroxyl and 4-oxo groups on their "flavonol backbone," which contribute to the specific enzyme-substrate interaction. While hPirin is iron-dependent, YhhW is identified as a novel nickel-containing dioxygenase member of the bicupin family. Besides the expanded Pirin activity, we present the crystal structures of the native Ni-YhhW and tag-free Fe-hPirin, revealing the distinctive differences occurring at the metal-binding site. In addition, YhhW features a flexible Ω-loop near the catalytic cavity, which may help stabilize the reaction intermediates via a Ni-flavonol complex. The structure-dependent modulation of substrate binding to the catalytic cavity adds to understanding the differential dispositions of natural flavonols by human and bacterial Pirins.
中文翻译:

Pirin蛋白对植物黄酮醇的底物结合和生物降解活性的结构依赖性调节。
Pirin是一种非血红素金属蛋白,广泛存在于人体组织中,并且在所有分类单元中都高度保守。Pirin蛋白通常起核转录调节剂的作用,但揭示了两个Pirin直系同源物YhhW(来自大肠杆菌)和hPirin(来自人类)具有降解槲皮素的酶活性。皮林同系物的确切作用及其催化特异性仍然知之甚少。在这项工作中,通过针对一组植物类黄酮进行筛选,我们发现两种Pirins均可催化多种类黄酮醇类似物的氧化降解,并在此过程中释放一氧化碳(CO)。这表明Pirin可以作用于多种底物,并且可以代表体内一氧化碳的新型饮食来源。尽管两个Pirin之间的动力学曲线存在显着差异,鉴定出的底物结构在其“黄酮醇主链”上均共享2,3-双键以及3-羟基和4-氧代基团,这有助于特定的酶-底物相互作用。虽然hPirin是铁依赖性的,但YhhW被确定为bicupin家族的新型含镍双加氧酶成员。除了扩展的Pirin活性,我们还展示了天然Ni-YhhW和无标签的Fe-hPirin的晶体结构,揭示了在金属结合位点发生的独特差异。此外,YhhW在催化腔附近具有一个柔性Ω环,可通过镍-黄酮醇配合物帮助稳定反应中间体。底物结合至催化腔的结构依赖性调节增加了对人和细菌Pirin对天然黄酮醇的不同处置的了解。
更新日期:2019-10-24
中文翻译:

Pirin蛋白对植物黄酮醇的底物结合和生物降解活性的结构依赖性调节。
Pirin是一种非血红素金属蛋白,广泛存在于人体组织中,并且在所有分类单元中都高度保守。Pirin蛋白通常起核转录调节剂的作用,但揭示了两个Pirin直系同源物YhhW(来自大肠杆菌)和hPirin(来自人类)具有降解槲皮素的酶活性。皮林同系物的确切作用及其催化特异性仍然知之甚少。在这项工作中,通过针对一组植物类黄酮进行筛选,我们发现两种Pirins均可催化多种类黄酮醇类似物的氧化降解,并在此过程中释放一氧化碳(CO)。这表明Pirin可以作用于多种底物,并且可以代表体内一氧化碳的新型饮食来源。尽管两个Pirin之间的动力学曲线存在显着差异,鉴定出的底物结构在其“黄酮醇主链”上均共享2,3-双键以及3-羟基和4-氧代基团,这有助于特定的酶-底物相互作用。虽然hPirin是铁依赖性的,但YhhW被确定为bicupin家族的新型含镍双加氧酶成员。除了扩展的Pirin活性,我们还展示了天然Ni-YhhW和无标签的Fe-hPirin的晶体结构,揭示了在金属结合位点发生的独特差异。此外,YhhW在催化腔附近具有一个柔性Ω环,可通过镍-黄酮醇配合物帮助稳定反应中间体。底物结合至催化腔的结构依赖性调节增加了对人和细菌Pirin对天然黄酮醇的不同处置的了解。

































 京公网安备 11010802027423号
京公网安备 11010802027423号