Synthesis ( IF 2.2 ) Pub Date : 2019-10-14 , DOI: 10.1055/s-0037-1610732 Fang Li , Feifei He , Rene M. Koenigs 1
|
Abstract
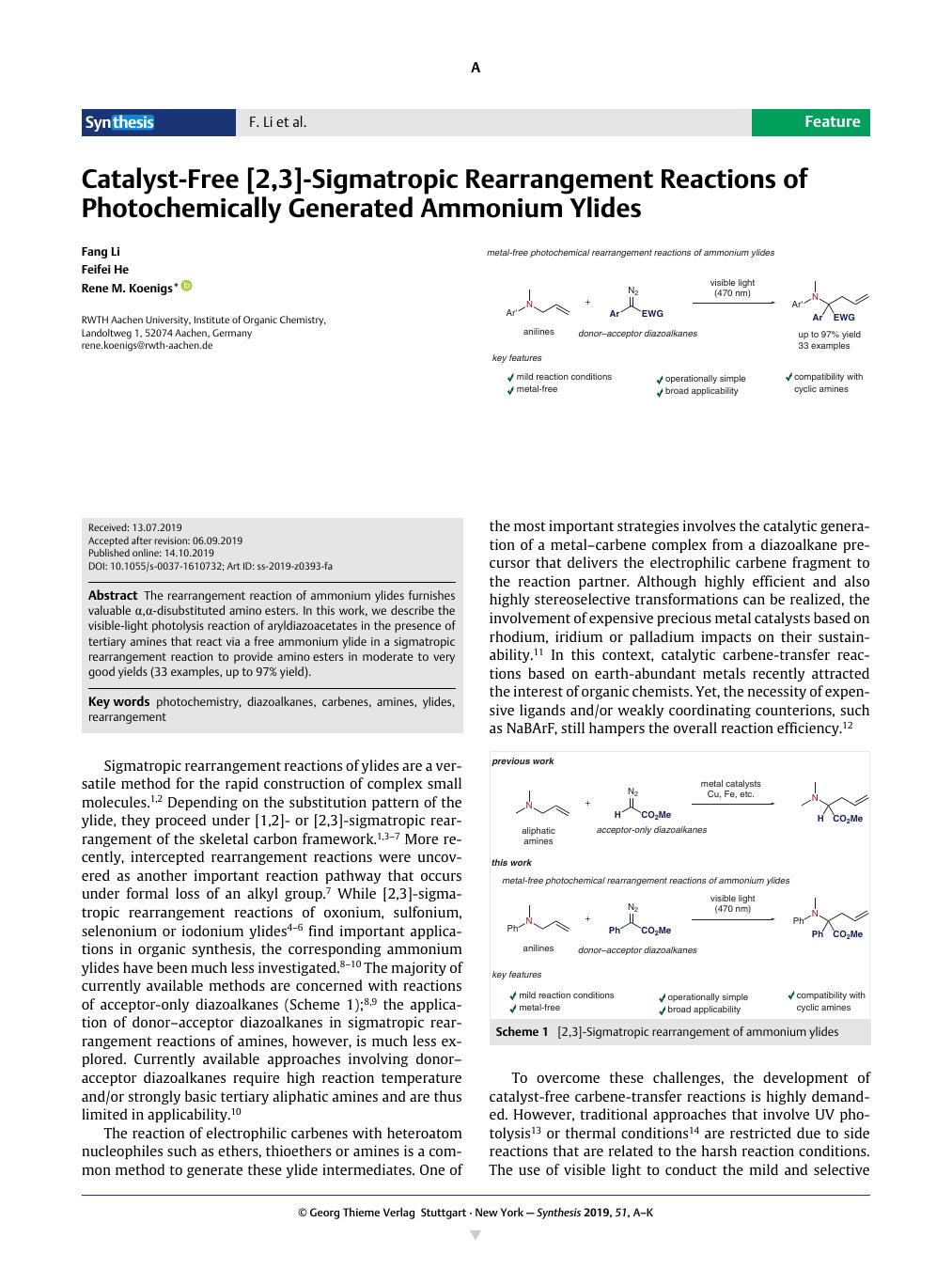
The rearrangement reaction of ammonium ylides furnishes valuable α,α-disubstituted amino esters. In this work, we describe the visible-light photolysis reaction of aryldiazoacetates in the presence of tertiary amines that react via a free ammonium ylide in a sigmatropic rearrangement reaction to provide amino esters in moderate to very good yields (33 examples, up to 97% yield).
The rearrangement reaction of ammonium ylides furnishes valuable α,α-disubstituted amino esters. In this work, we describe the visible-light photolysis reaction of aryldiazoacetates in the presence of tertiary amines that react via a free ammonium ylide in a sigmatropic rearrangement reaction to provide amino esters in moderate to very good yields (33 examples, up to 97% yield).
中文翻译:

光化学生成的铵盐化物的无催化剂[2,3]-σ重排反应
抽象的
铵盐的重排反应提供了有价值的α,α-二取代的氨基酯。在这项工作中,我们描述了存在叔胺的情况下芳基重氮乙酸酯的可见光光解反应,该叔胺通过游离的叶立德铵在σ重排反应中反应,以中等至非常高的产率提供氨基酯(33个实例,最高可达97%屈服)。
铵盐的重排反应提供了有价值的α,α-二取代的氨基酯。在这项工作中,我们描述了存在叔胺的情况下芳基重氮乙酸酯的可见光光解反应,该叔胺通过游离的叶立德铵在σ重排反应中反应,以中等至非常高的产率提供氨基酯(33个实例,最高可达97%屈服)。































 京公网安备 11010802027423号
京公网安备 11010802027423号