Colloids and Surfaces B: Biointerfaces ( IF 5.4 ) Pub Date : 2019-10-13 , DOI: 10.1016/j.colsurfb.2019.110572 Sashini S Wijetunge 1 , Jianchuan Wen 1 , Chih-Ko Yeh 2 , Yuyu Sun 1
|
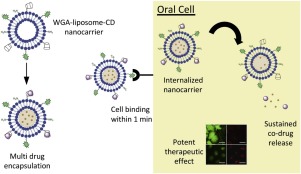
Topical management of oral infection requires combined use of multiple classes of drugs and frequent dosing due to low drug retention rates. The sustained, co-delivery of drugs with different solubilities to cells using nanoparticle drug delivery systems remains a challenge. Here, we developed wheat germ agglutinin (WGA) conjugated liposomes with surface grafted cyclodextrin (WGA-liposome-CD) as bioadhesive dual-drug nanocarriers. We effectively encapsulated two physiochemically different drugs (ciprofloxacin and betamethasone) and demonstrated sustained co-drug release in saliva over a 24 h period in vitro. As proof of therapeutic utility in oral cells, we infected oral keratinocytes with Aggregatibacter actinomycetemcomitans, a bacterial pathogen responsible for chronic periodontal disease. Drug release, resulting from nanocarrier cell binding, produced a significant increase in oral cell survival and synergistically reduced inflammation. These results suggest that WGA-liposome-CD nanocarriers are novel cyto-adhesive candidates for delivering multiple drugs with sustained therapeutic activity for localized drug delivery to oral cells.
中文翻译:

小麦胚芽凝集素脂质体与表面嫁接的环糊精作为生物粘附性双药递送纳米载体,用于治疗口腔细胞。
口服感染的局部治疗由于药物保留率低,需要联合使用多种药物和频繁给药。使用纳米颗粒药物递送系统将具有不同溶解度的药物持续共递送至细胞仍然是一个挑战。在这里,我们开发了小麦胚芽凝集素(WGA)缀合的脂质体与表面嫁接的环糊精(WGA-脂质体-CD)作为生物粘附双药物纳米载体。我们有效地封装了两种理化性质不同的药物(环丙沙星和倍他米松),并在体外24小时内证明了唾液中持续的共同药物释放。作为在口腔细胞中治疗作用的证据,我们用聚合放线菌放线菌感染了口腔角质形成细胞,一种引起慢性牙周疾病的细菌病原体。由纳米载体细胞结合导致的药物释放大大增加了口腔细胞的存活率,并协同减少了炎症。这些结果表明,WGA-脂质体-CD纳米载体是新型的细胞粘附候选物,可用于将多种药物具有持久的治疗活性,从而将药物局部递送至口腔细胞。






























 京公网安备 11010802027423号
京公网安备 11010802027423号