Cell Death & Disease ( IF 8.1 ) Pub Date : 2019-10-14 , DOI: 10.1038/s41419-019-2021-3 Chang Jia 1 , Jian Zhang 2 , Huanwen Chen 3 , Yingzhi Zhuge 2 , Huiqiao Chen 2 , Fanyu Qian 2 , Kailiang Zhou 4 , Chao Niu 1 , Fangyan Wang 5 , Huixian Qiu 2 , Zhenquan Wang 2 , Jian Xiao 6 , Xing Rong 2 , Maoping Chu 1, 2
|
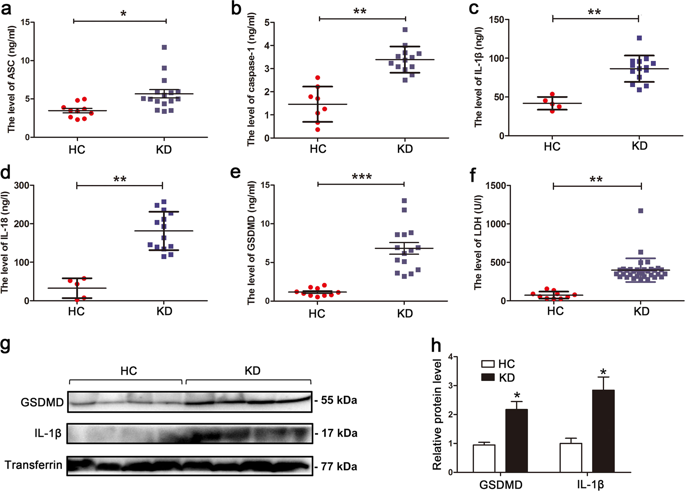
Kawasaki disease (KD) is the most common cause of pediatric cardiac disease in developed countries, and can lead to permanent coronary artery damage and long term sequelae such as coronary artery aneurysms. Given the prevalence and severity of KD, further research is warranted on its pathophysiology. It is known that endothelial cell damage and inflammation are two essential processes resulting in the coronary endothelial dysfunction in KD. However, detailed mechanisms are largely unknown. In this study, we investigated the role of pyroptosis in the setting of KD, and hypothesized that pyroptosis may play a central role in its pathophysiology. In vivo experiments of patients with KD demonstrated that serum levels of pyroptosis-related proteins, including ASC, caspase-1, IL-1β, IL-18, GSDMD and lactic dehydrogenase (LDH), were significantly increased in KD compared with healthy controls (HCs). Moreover, western blot analysis showed that the expression of GSDMD and mature IL-1β was notably elevated in KD sera. In vitro, exposure of human umbilical vein endothelial cells (HUVECs) to KD sera-treated THP1 cells resulted in the activation of NLRP3 inflammasome and subsequent pyroptosis induction, as evidenced by elevated expression of caspase-1, GSDMD, cleaved p30 form of GSDMD, IL-1β and IL-18, and increased LDH release and TUNEL and propidium iodide (PI)-positive cells. Furthermore, our results showed that NLRP3-dependent endothelial cell pyroptosis was activated by HMGB1/RAGE/cathepsin B signaling. These findings were also recapitulated in a mouse model of KD induced by Candida albicans cell wall extracts (CAWS). Together, our findings suggest that endothelial cell pyroptosis may play a significant role in coronary endothelial damage in KD, providing novel evidence that further elucidates its pathophysiology.
中文翻译:

内皮细胞热凋亡通过HMGB1 / RAGE / cathespin B信号通路和NLRP3炎性体激活在川崎病中起重要作用。
川崎病(KD)是发达国家小儿心脏病的最常见病因,可导致永久性冠状动脉损害和长期后遗症,例如冠状动脉瘤。考虑到KD的患病率和严重性,有必要对其病理生理学进行进一步研究。众所周知,内皮细胞损伤和炎症是导致KD冠状动脉内皮功能障碍的两个基本过程。但是,详细的机制在很大程度上是未知的。在这项研究中,我们调查了细胞凋亡在KD病情中的作用,并假设细胞凋亡可能在其病理生理学中起着核心作用。对KD患者进行的体内实验表明,血清中与凋亡相关的蛋白质(包括ASC,caspase-1,IL-1β,IL-18,GSMDD和乳酸脱氢酶(LDH))的水平 与健康对照组(HCs)相比,KD显着增加。此外,蛋白质印迹分析表明,KSD血清中GSDMD和成熟IL-1β的表达显着升高。在体外,将人脐静脉内皮细胞(HUVEC)暴露于KD血清处理过的THP1细胞会导致NLRP3炎性小体的活化和随后的光化诱导,这由caspase-1,GSMDD,gSDMD的p30裂解形式的表达升高证明了这一点, IL-1β和IL-18,以及增加的LDH释放以及TUNEL和碘化丙啶(PI)阳性细胞。此外,我们的结果表明,HMGB1 / RAGE /组织蛋白酶B信号激活了依赖NLRP3的内皮细胞凋亡。这些发现在由KD诱导的KD小鼠模型中也得到了概括。免疫印迹分析表明,KSD血清中GSDMD和成熟IL-1β的表达显着升高。在体外,将人脐静脉内皮细胞(HUVEC)暴露于KD血清处理的THP1细胞会导致NLRP3炎性小体活化,并随后引起凋亡诱导,这由caspase-1,GSMDD,gSDMD的p30裂解形式的表达升高证明了这一点, IL-1β和IL-18,以及增加的LDH释放以及TUNEL和碘化丙啶(PI)阳性细胞。此外,我们的结果表明,HMGB1 / RAGE /组织蛋白酶B信号激活了依赖NLRP3的内皮细胞凋亡。这些发现在由KD诱导的KD小鼠模型中也得到了概括。免疫印迹分析表明,KSD血清中GSDMD和成熟IL-1β的表达显着升高。在体外,将人脐静脉内皮细胞(HUVEC)暴露于KD血清处理的THP1细胞会导致NLRP3炎性小体活化,并随后引起凋亡诱导,这由caspase-1,GSMDD,gSDMD的p30裂解形式的表达升高证明了这一点, IL-1β和IL-18,以及增加的LDH释放以及TUNEL和碘化丙啶(PI)阳性细胞。此外,我们的结果表明,HMGB1 / RAGE /组织蛋白酶B信号激活了依赖NLRP3的内皮细胞凋亡。这些发现在由KD诱导的KD小鼠模型中也得到了概括。将人脐静脉内皮细胞(HUVEC)暴露于KD血清处理的THP1细胞会导致NLRP3炎性小体活化,并随后引起凋亡诱导,这可通过caspase-1,GSMDD,pSD 30形式的GSDMD,IL-1β裂解表达来证明。和IL-18,以及增加的LDH释放以及TUNEL和碘化丙啶(PI)阳性细胞。此外,我们的结果表明,HMGB1 / RAGE /组织蛋白酶B信号激活了依赖NLRP3的内皮细胞凋亡。这些发现在由KD诱导的KD小鼠模型中也得到了概括。将人脐静脉内皮细胞(HUVEC)暴露于KD血清处理的THP1细胞会导致NLRP3炎性小体活化,并随后引起凋亡诱导,这可通过caspase-1,GSMDD,pSD 30形式的GSDMD,IL-1β裂解表达来证明。和IL-18,以及增加的LDH释放以及TUNEL和碘化丙啶(PI)阳性细胞。此外,我们的结果表明,HMGB1 / RAGE /组织蛋白酶B信号激活了依赖NLRP3的内皮细胞凋亡。这些发现在由KD诱导的KD小鼠模型中也得到了概括。我们的结果表明,HMGB1 / RAGE /组织蛋白酶B信号激活了依赖NLRP3的内皮细胞凋亡。这些发现在由KD诱导的KD小鼠模型中也得到了概括。我们的结果表明,HMGB1 / RAGE /组织蛋白酶B信号激活了依赖NLRP3的内皮细胞凋亡。这些发现在由KD诱导的KD小鼠模型中也得到了概括。白色念珠菌细胞壁提取物(CAWS)。在一起,我们的发现表明,内皮细胞的热解可能在KD的冠状动脉内皮损伤中起重要作用,提供了进一步阐明其病理生理学的新证据。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号