当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chiral‐at‐Metal Rhodium(III) Complex Catalyzed Enantioselective Vinylogous Michael Addition of α,α‐Dicyanoolefins with α,β‐Unsaturated 2‐Acyl Imidazoles
ChemCatChem ( IF 3.8 ) Pub Date : 2019-11-26 , DOI: 10.1002/cctc.201901590 Liangjian Hu 1 , Shaoxia Lin 2 , Shiwu Li 2 , Qiang Kang 2 , Yu Du 2
ChemCatChem ( IF 3.8 ) Pub Date : 2019-11-26 , DOI: 10.1002/cctc.201901590 Liangjian Hu 1 , Shaoxia Lin 2 , Shiwu Li 2 , Qiang Kang 2 , Yu Du 2
Affiliation
|
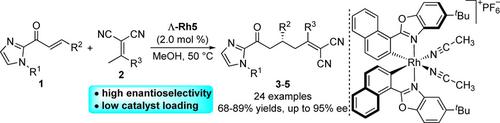
As a development of traditional vinylogous Michael addition reactions, catalytic enantioselective γ‐functionalization of α,α‐dicyanoolefins possesses a remarkable advantage in carboxylic derivatives synthesis and has emerged as a powerful tool for the preparation of high value chemicals. In another hand, the development of versatile and highly efficient catalyst is quite critical for such asymmetric transformations. Herein, a newly developed chiral‐at‐metal rhodium (III) complex catalyzed enantioselective vinylogous Michael addition of α,α‐dicyanoolefins with α,β‐unsaturated 2‐acyl imidazoles has been realized, delivering the corresponding adducts in 68−89 % yields with up to 95 % enantioselectivity. The reaction can be conducted on a gram‐scale using a low catalyst loading (0.5 mol %) without impacting its efficiency.
中文翻译:

手性金属铑(III)配合物催化α,α-二氰基烯烃与α,β-不饱和2-酰基咪唑的对映选择性乙烯基加成反应
由于传统的插烯迈克尔加成反应的发展,对映选择性催化γ的-functionalization α,α -dicyanoolefins拥有显着的优点在羧酸衍生物的合成和已成为用于高价值化学品制备的有力工具。另一方面,对于这种不对称转化而言,通用和高效催化剂的开发是至关重要的。本文中,新开发的手性金属铑(III)配合物催化α,α-二氰基烯烃与α,β的对映选择性乙烯基迈克尔加成已经实现了不饱和的2-酰基咪唑,以68-89%的产率提供了相应的加合物,对映选择性高达95%。该反应可以在不影响反应效率的情况下,以低催化剂用量(0.5 mol%)以克为单位进行。
更新日期:2019-11-27
中文翻译:

手性金属铑(III)配合物催化α,α-二氰基烯烃与α,β-不饱和2-酰基咪唑的对映选择性乙烯基加成反应
由于传统的插烯迈克尔加成反应的发展,对映选择性催化γ的-functionalization α,α -dicyanoolefins拥有显着的优点在羧酸衍生物的合成和已成为用于高价值化学品制备的有力工具。另一方面,对于这种不对称转化而言,通用和高效催化剂的开发是至关重要的。本文中,新开发的手性金属铑(III)配合物催化α,α-二氰基烯烃与α,β的对映选择性乙烯基迈克尔加成已经实现了不饱和的2-酰基咪唑,以68-89%的产率提供了相应的加合物,对映选择性高达95%。该反应可以在不影响反应效率的情况下,以低催化剂用量(0.5 mol%)以克为单位进行。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号