European Journal of Pharmaceutics and Biopharmaceutics ( IF 4.4 ) Pub Date : 2018-03-06 , DOI: 10.1016/j.ejpb.2018.03.003 Jacqueline Horn , Julia Schanda , Wolfgang Friess
|
Purpose
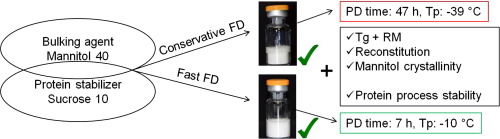
Mannitol/sucrose formulations are employed to generate lyophilizates for biopharmaceuticals with an elegant cake appearance. The aim of this study was to dry protein/mannitol/sucrose formulations as fast as possible without loss of cake appearance and protein stability. Glycerol was included as potential additional protein stabilizer. Three proteins (lysozyme and two monoclonal antibodies) at low and high concentration were analyzed comparing fast with conservative freeze-drying.
Methods
Freeze-drying cycle development was carried out with mannitol/sucrose formulations. A product temperature (Tp) close to the Te of mannitol and clearly above the Tg’ of sucrose was targeted. Protein formulations were exposed to the final fast lyophilisation process and to a conservative freeze-drying cycle. Lyophilizates were characterized by differential scanning calorimetry, Karl-Fischer titration and X-ray diffractometry. Additionally, macroscopic cake appearance and reconstitution times were evaluated. Protein stability was characterized by UV/Vis spectroscopy, light obscuration and size exclusion chromatography.
Results
The fast freeze-drying cycle resulted in a primary drying time of 7 h (Tp: −10 °C) and a secondary drying time of 2 h in contrast to 47 h (Tp: −39 °C) and 12 h for the conservative cycle. Lyophilizates showed Tg values above 60 °C, a residual moisture level of 1%, reconstitution times of less than 35 s, δ-mannitol and elegant cake appearance. Mannitol/sucrose ratios below 4/1 did not lead to complete mannitol crystallization and were therefore not suitable for the selected process conditions. Characterisation of protein stability rendered low aggregation and particle levels for both, fast and conservative freeze-drying conditions.
Conclusions
It was shown that fast freeze-drying of mannitol/sucrose formulations above Tg’ at a Tp of −10 °C resulted in good protein process stability and appropriate cake characteristics at maximum time reduction.
中文翻译:

快速保守冷冻干燥对蛋白质-甘露醇-蔗糖-甘油冻干粉产品质量的影响
目的
甘露醇/蔗糖制剂用于产生具有优雅蛋糕外观的生物药物冻干剂。这项研究的目的是尽可能快地干燥蛋白质/甘露醇/蔗糖配方,而不会损失蛋糕外观和蛋白质稳定性。甘油被包括作为潜在的附加蛋白质稳定剂。分析了低浓度和高浓度的三种蛋白质(溶菌酶和两种单克隆抗体),并与快速冷冻干燥法进行了比较。
方法
用甘露醇/蔗糖制剂进行冷冻干燥循环显影。以接近甘露醇Te且明显高于蔗糖Tg'的产物温度(Tp)为目标。将蛋白质制剂暴露于最终的快速冻干过程和保守的冷冻干燥循环中。冻干物通过差示扫描量热法,Karl-Fischer滴定法和X射线衍射法表征。另外,评估了宏观蛋糕外观和重构时间。通过UV / Vis光谱,光遮蔽和尺寸排阻色谱法表征蛋白质稳定性。
结果
快速的冷冻干燥周期导致一次干燥时间为7小时(Tp:-10°C)和二次干燥时间为2 h,而传统干燥时间为47 h(Tp:−39°C),保守干燥时间为12 h循环。冻干物显示Tg值高于60°C,残留水分含量为1%,重构时间少于35 s,δ-甘露醇和精美的蛋糕外观。低于4/1的甘露醇/蔗糖比例不能导致甘露醇完全结晶,因此不适合所选的工艺条件。蛋白质稳定性的表征使得快速和保守冷冻干燥条件下的聚集度和颗粒水平都较低。
结论
结果表明,在-10°C的Tp下,高于Tg'的甘露醇/蔗糖制剂的快速冷冻干燥导致了良好的蛋白质加工稳定性,并在最大的时间内减少了适当的饼状特性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号