当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aminothiazoles and aminothiadiazoles as nucleophiles in aminocarbonylation of iodobenzene derivatives
Tetrahedron ( IF 2.1 ) Pub Date : 2018-03-06 , DOI: 10.1016/j.tet.2018.03.007 Máté Gergely , László Kollár
中文翻译:

氨基噻唑和氨基噻二唑作为碘苯衍生物氨基羰基化中的亲核试剂
更新日期:2018-03-06
Tetrahedron ( IF 2.1 ) Pub Date : 2018-03-06 , DOI: 10.1016/j.tet.2018.03.007 Máté Gergely , László Kollár
|
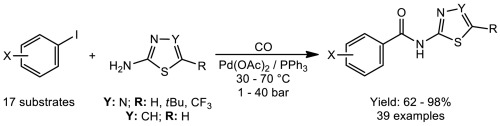
Various 2-, 3- and 4-substituted iodobenzenes were aminocarbonylated using aminothiazole and aminothiadiazole derivatives in palladium-catalysed reaction. The reaction is chemospecific toward the corresponding carboxamides. Consequently, the application of the above N-nucleophiles provided the N-1,3-thiazol-2-yl- and N-1,3,4-thiadiazol-2-ylcarboxamides in moderate to high yields. Due to the facile work-up of the reaction mixture isolated yields of 90% or higher were obtained in most cases.
中文翻译:

氨基噻唑和氨基噻二唑作为碘苯衍生物氨基羰基化中的亲核试剂
使用氨基噻唑和氨基噻二唑衍生物在钯催化的反应中将各种2-,3-和4-取代的碘苯氨基羰基化。该反应对相应的羧酰胺具有化学特异性。因此,上述N-亲核试剂的应用以中等至高产率提供了N -1,3-噻唑-2-基-和N -1,3,4-噻二唑-2-基甲酰胺。由于反应混合物易于后处理,在大多数情况下,分离出的产率为90%或更高。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号