当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
RORγ is a targetable master regulator of cholesterol biosynthesis in a cancer subtype.
Nature Communications ( IF 14.7 ) Pub Date : 2019-10-11 , DOI: 10.1038/s41467-019-12529-3
Demin Cai 1 , Junjian Wang 1 , Bei Gao 2 , Jin Li 1 , Feng Wu 3 , June X Zou 1 , Jianzhen Xu 4 , Yuqian Jiang 1 , Hongye Zou 1 , Zenghong Huang 1 , Alexander D Borowsky 5 , Richard J Bold 6, 7 , Primo N Lara 7 , Jian Jian Li 8 , Xinbin Chen 7, 9 , Kit S Lam 1, 7 , Ka-Fai To 3 , Hsing-Jien Kung 1, 7 , Oliver Fiehn 2 , Ruqian Zhao 10, 11 , Ronald M Evans 12 , Hong-Wu Chen 1, 7
Nature Communications ( IF 14.7 ) Pub Date : 2019-10-11 , DOI: 10.1038/s41467-019-12529-3
Demin Cai 1 , Junjian Wang 1 , Bei Gao 2 , Jin Li 1 , Feng Wu 3 , June X Zou 1 , Jianzhen Xu 4 , Yuqian Jiang 1 , Hongye Zou 1 , Zenghong Huang 1 , Alexander D Borowsky 5 , Richard J Bold 6, 7 , Primo N Lara 7 , Jian Jian Li 8 , Xinbin Chen 7, 9 , Kit S Lam 1, 7 , Ka-Fai To 3 , Hsing-Jien Kung 1, 7 , Oliver Fiehn 2 , Ruqian Zhao 10, 11 , Ronald M Evans 12 , Hong-Wu Chen 1, 7
Affiliation
|
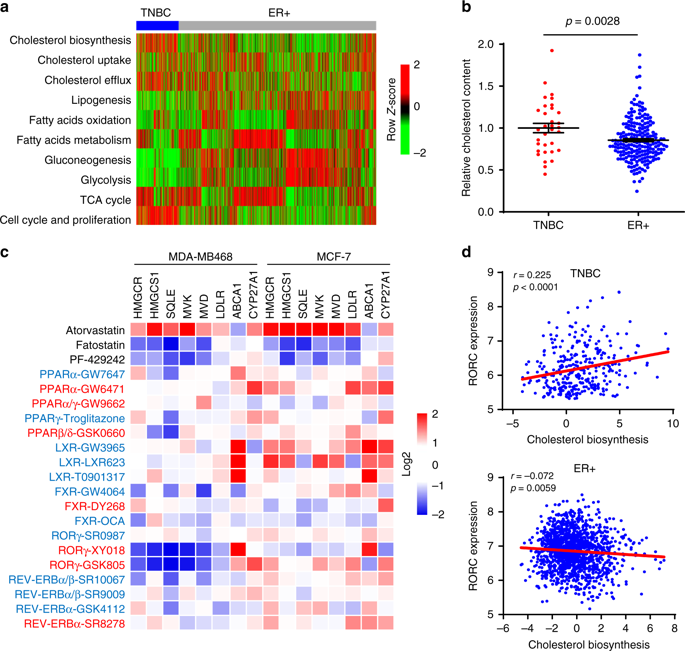
Tumor subtype-specific metabolic reprogrammers could serve as targets of therapeutic intervention. Here we show that triple-negative breast cancer (TNBC) exhibits a hyper-activated cholesterol-biosynthesis program that is strongly linked to nuclear receptor RORγ, compared to estrogen receptor-positive breast cancer. Genetic and pharmacological inhibition of RORγ reduces tumor cholesterol content and synthesis rate while preserving host cholesterol homeostasis. We demonstrate that RORγ functions as an essential activator of the entire cholesterol-biosynthesis program, dominating SREBP2 via its binding to cholesterol-biosynthesis genes and its facilitation of the recruitment of SREBP2. RORγ inhibition disrupts its association with SREBP2 and reduces chromatin acetylation at cholesterol-biosynthesis gene loci. RORγ antagonists cause tumor regression in patient-derived xenografts and immune-intact models. Their combination with cholesterol-lowering statins elicits superior anti-tumor synergy selectively in TNBC. Together, our study uncovers a master regulator of the cholesterol-biosynthesis program and an attractive target for TNBC.
中文翻译:

RORγ 是癌症亚型中胆固醇生物合成的靶向主调节因子。
肿瘤亚型特异性代谢重编程器可以作为治疗干预的靶点。在这里,我们表明,与雌激素受体阳性乳腺癌相比,三阴性乳腺癌 (TNBC) 表现出与核受体 RORγ 密切相关的过度激活的胆固醇生物合成程序。RORγ 的遗传和药理学抑制降低了肿瘤胆固醇含量和合成速率,同时保持了宿主胆固醇稳态。我们证明 RORγ 作为整个胆固醇生物合成程序的重要激活剂发挥作用,通过与胆固醇生物合成基因的结合和促进 SREBP2 的募集来控制 SREBP2。RORγ 抑制破坏其与 SREBP2 的结合,并减少胆固醇生物合成基因位点的染色质乙酰化。RORγ 拮抗剂在患者来源的异种移植物和免疫完整模型中导致肿瘤消退。它们与降低胆固醇的他汀类药物的结合在 TNBC 中选择性地引发卓越的抗肿瘤协同作用。总之,我们的研究揭示了胆固醇生物合成程序的主要调节因子和 TNBC 的一个有吸引力的靶标。
更新日期:2019-10-12
中文翻译:

RORγ 是癌症亚型中胆固醇生物合成的靶向主调节因子。
肿瘤亚型特异性代谢重编程器可以作为治疗干预的靶点。在这里,我们表明,与雌激素受体阳性乳腺癌相比,三阴性乳腺癌 (TNBC) 表现出与核受体 RORγ 密切相关的过度激活的胆固醇生物合成程序。RORγ 的遗传和药理学抑制降低了肿瘤胆固醇含量和合成速率,同时保持了宿主胆固醇稳态。我们证明 RORγ 作为整个胆固醇生物合成程序的重要激活剂发挥作用,通过与胆固醇生物合成基因的结合和促进 SREBP2 的募集来控制 SREBP2。RORγ 抑制破坏其与 SREBP2 的结合,并减少胆固醇生物合成基因位点的染色质乙酰化。RORγ 拮抗剂在患者来源的异种移植物和免疫完整模型中导致肿瘤消退。它们与降低胆固醇的他汀类药物的结合在 TNBC 中选择性地引发卓越的抗肿瘤协同作用。总之,我们的研究揭示了胆固醇生物合成程序的主要调节因子和 TNBC 的一个有吸引力的靶标。

































 京公网安备 11010802027423号
京公网安备 11010802027423号