当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical CO2 Reduction to C1 Products on Single Nickel/Cobalt/Iron-Doped Graphitic Carbon Nitride: A DFT Study.
ChemSusChem ( IF 7.5 ) Pub Date : 2019-11-14 , DOI: 10.1002/cssc.201902483 Chen Guo 1 , Tian Zhang 2 , Xiangxuan Deng 1 , Xiongyi Liang 1 , Wenyue Guo 2 , Xiaoqing Lu 2 , Chi-Man Lawrence Wu 1
ChemSusChem ( IF 7.5 ) Pub Date : 2019-11-14 , DOI: 10.1002/cssc.201902483 Chen Guo 1 , Tian Zhang 2 , Xiangxuan Deng 1 , Xiongyi Liang 1 , Wenyue Guo 2 , Xiaoqing Lu 2 , Chi-Man Lawrence Wu 1
Affiliation
|
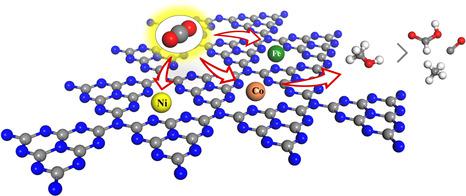
Electrocatalytic CO2 reduction reaction (CRR) is one of the most promising strategies to convert greenhouse gases to energy sources. Herein, the CRR was applied towards making C1 products (CO, HCOOH, CH3 OH, and CH4 ) on g-C3 N4 frameworks with single Ni, Co, and Fe introduction; this process was investigated by density functional theory. The structures of the electrocatalysts, CO2 adsorption configurations, and CO2 reduction mechanisms were systematically studied. Results showed that the single Ni, Co, and Fe located from the corner of the g-C3 N4 cavity to the center. Analyses of the adsorption configurations and electronic structures suggested that CO2 could be chemically adsorbed on Co-C3 N4 and Fe-C3 N4 , but physically adsorbed on Ni-C3 N4 . The H2 evolution reaction (HER), as a suppression of CRR, was investigated, and results showed that Ni-C3 N4 , Co-C3 N4 , and Fe-C3 N4 exhibited more CRR selectivity than HER. CRR proceeded via COOH and OCHO as initial protonation intermediates on Ni-C3 N4 and Co/Fe-C3 N4 , respectively, which resulted in different C1 products along quite different reaction pathways. Compared with Ni-C3 N4 and Fe-C3 N4 , Co-C3 N4 had more favorable CRR activity and selectivity for CH3 OH production with unique rate-limiting steps and lower limiting potential.
中文翻译:

DFT研究:在单镍/钴/铁掺杂的石墨碳氮化物上电化学还原CO2生成C1产品。
电催化还原二氧化碳(CRR)是将温室气体转化为能源的最有希望的策略之一。本文中,CRR用于在单一引入Ni,Co和Fe的g-C3 N4框架上制备C1产物(CO,HCOOH,CH3 OH和CH4)。通过密度泛函理论研究了该过程。系统地研究了电催化剂的结构,CO 2吸附构型和CO 2还原机理。结果表明,单一的Ni,Co和Fe从g-C3 N4腔的角到中心。吸附构型和电子结构分析表明,CO2可以化学吸附在Co-C3 N4和Fe-C3 N4上,但可以物理吸附在Ni-C3 N4上。研究了H2放出反应(HER)作为抑制CRR的方法,结果表明,Ni-C3 N4,Co-C3 N4和Fe-C3 N4比HER表现出更高的CRR选择性。CRR分别通过COOH和OCHO作为Ni-C3 N4和Co / Fe-C3 N4上的初始质子化中间体进行,导致沿完全不同的反应路径产生不同的C1产物。与Ni-C3 N4和Fe-C3 N4相比,Co-C3 N4具有更有利的CRR活性和选择性,具有独特的限速步骤和较低的限制电位,可用于CH3 OH的生产。
更新日期:2019-11-15
中文翻译:

DFT研究:在单镍/钴/铁掺杂的石墨碳氮化物上电化学还原CO2生成C1产品。
电催化还原二氧化碳(CRR)是将温室气体转化为能源的最有希望的策略之一。本文中,CRR用于在单一引入Ni,Co和Fe的g-C3 N4框架上制备C1产物(CO,HCOOH,CH3 OH和CH4)。通过密度泛函理论研究了该过程。系统地研究了电催化剂的结构,CO 2吸附构型和CO 2还原机理。结果表明,单一的Ni,Co和Fe从g-C3 N4腔的角到中心。吸附构型和电子结构分析表明,CO2可以化学吸附在Co-C3 N4和Fe-C3 N4上,但可以物理吸附在Ni-C3 N4上。研究了H2放出反应(HER)作为抑制CRR的方法,结果表明,Ni-C3 N4,Co-C3 N4和Fe-C3 N4比HER表现出更高的CRR选择性。CRR分别通过COOH和OCHO作为Ni-C3 N4和Co / Fe-C3 N4上的初始质子化中间体进行,导致沿完全不同的反应路径产生不同的C1产物。与Ni-C3 N4和Fe-C3 N4相比,Co-C3 N4具有更有利的CRR活性和选择性,具有独特的限速步骤和较低的限制电位,可用于CH3 OH的生产。































 京公网安备 11010802027423号
京公网安备 11010802027423号