当前位置:
X-MOL 学术
›
Spectrochim. Acta. A Mol. Biomol. Spectrosc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Development of sulfur doped carbon quantum dots for highly selective and sensitive fluorescent detection of Fe2+ and Fe3+ ions in oral ferrous gluconate samples.
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.3 ) Pub Date : 2019-10-08 , DOI: 10.1016/j.saa.2019.117602 Fuyou Du 1 , Zhenfang Cheng 2 , Wei Tan 3 , Lingshun Sun 2 , Guihua Ruan 2
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.3 ) Pub Date : 2019-10-08 , DOI: 10.1016/j.saa.2019.117602 Fuyou Du 1 , Zhenfang Cheng 2 , Wei Tan 3 , Lingshun Sun 2 , Guihua Ruan 2
Affiliation
|
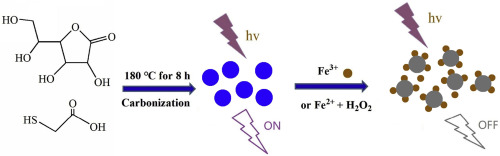
Sulfur-doped carbon quantum dots (S-CQDs) with stable blue fluorescence were synthesized through a facile one-step hydrothermal method by using ascorbic acid and thioglycolic acid as carbon and sulfur sources. The prepared S-CQDs exhibited a sensitive and selective response to Fe3+ ions in comparison with Fe2+ and other metal ions, In the presence of adequate H2O2, Fe2+ was completely transformed to Fe3+ that is the determinable form of iron ions, and the difference in the change of the fluorescence intensity of S-CQDs before and after adding H2O2 was used for detection of Fe2+ and Fe3+ ions, respectively. Under the optimum experimental conditions, the fluorescence intensity of S-CQDs gradually decreased with increasing of Fe3+ concentration ranging from 0 to 200 μM. Good linearity was achieved over the range of 0-200 μM. The detection limit of the developed method was 0.050 μM for Fe3+. The recoveries of Fe2+ and Fe3+ spiked in real samples ranged from 98.2% to 112.4%. Finally, the proposed S-CQDs integrated with Fenton system was applied to the detection of Fe2+ and Fe3+ ions in oral ferrous gluconate samples, which presents potential applications in the speciation and determination of Fe2+ and Fe3+ ions in complex samples.
中文翻译:

开发了用于高选择性和灵敏荧光检测口腔葡萄糖酸亚铁样品中Fe2 +和Fe3 +离子的硫掺杂碳量子点。
以抗坏血酸和巯基乙酸为碳源和硫源,通过简便的一步水热法合成了具有稳定蓝色荧光的硫掺杂碳量子点(S-CQDs)。与Fe2 +和其他金属离子相比,制备的S-CQD对Fe3 +离子表现出敏感和选择性的反应。在存在足够的H2O2的情况下,Fe2 +完全转化为Fe3 +,这是铁离子的可确定形式,并且S-CQDs加入H2O2前后的荧光强度变化分别用于检测Fe2 +和Fe3 +离子。在最佳实验条件下,S-CQDs的荧光强度随着Fe3 +浓度从0到200μM的增加而逐渐降低。在0-200μM的范围内实现了良好的线性度。所开发方法对Fe3 +的检出限为0.050μM。实际样品中加标的Fe2 +和Fe3 +的回收率在98.2%至112.4%之间。最后,将拟议的与Fenton系统集成的S-CQDs用于口腔葡萄糖酸亚铁样品中Fe2 +和Fe3 +离子的检测,这在复杂样品中Fe2 +和Fe3 +离子的形态分析和测定中具有潜在的应用前景。
更新日期:2019-10-08
中文翻译:

开发了用于高选择性和灵敏荧光检测口腔葡萄糖酸亚铁样品中Fe2 +和Fe3 +离子的硫掺杂碳量子点。
以抗坏血酸和巯基乙酸为碳源和硫源,通过简便的一步水热法合成了具有稳定蓝色荧光的硫掺杂碳量子点(S-CQDs)。与Fe2 +和其他金属离子相比,制备的S-CQD对Fe3 +离子表现出敏感和选择性的反应。在存在足够的H2O2的情况下,Fe2 +完全转化为Fe3 +,这是铁离子的可确定形式,并且S-CQDs加入H2O2前后的荧光强度变化分别用于检测Fe2 +和Fe3 +离子。在最佳实验条件下,S-CQDs的荧光强度随着Fe3 +浓度从0到200μM的增加而逐渐降低。在0-200μM的范围内实现了良好的线性度。所开发方法对Fe3 +的检出限为0.050μM。实际样品中加标的Fe2 +和Fe3 +的回收率在98.2%至112.4%之间。最后,将拟议的与Fenton系统集成的S-CQDs用于口腔葡萄糖酸亚铁样品中Fe2 +和Fe3 +离子的检测,这在复杂样品中Fe2 +和Fe3 +离子的形态分析和测定中具有潜在的应用前景。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号