当前位置:
X-MOL 学术
›
J. Ind. Eng. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Microfluidic continuous flow synthesis of 1,5-ditosyl-1,5-diazocane-3,7-dione using response surface methodology
Journal of Industrial and Engineering Chemistry ( IF 5.9 ) Pub Date : 2020-02-01 , DOI: 10.1016/j.jiec.2019.10.002 Yang Zou , Tao Zhang , Guannan Wang , Mengwen Zhou , Yabo Xiong , Shaoyun Huang , Houbin Li , Xinghai Liu
Journal of Industrial and Engineering Chemistry ( IF 5.9 ) Pub Date : 2020-02-01 , DOI: 10.1016/j.jiec.2019.10.002 Yang Zou , Tao Zhang , Guannan Wang , Mengwen Zhou , Yabo Xiong , Shaoyun Huang , Houbin Li , Xinghai Liu
|
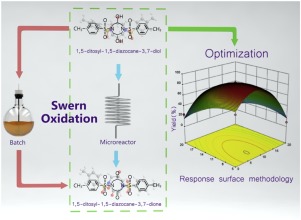
Abstract 3,3,7,7-tetrakis(difluoroamino) octahydro-1,5-dinitro-1,5-diazocine (HNFX) is a high-density energetic oxidizer with four difluoroamino groups (-NF2). In this study, 1,5-ditosyl-1,5-diazocane-3,7-dione, as an important intermediate for synthesis of HNFX, was successfully synthesized using Swern oxidation in a traditional batch reactor and a continuous flow microreactor, respectively. 1,5-dotosyl-1,5-diazocane-3,7-dione was characterized by HPLC, FTIR, 1H NMR, 13C NMR, Mass spectrometry, and X-ray crystal diffraction. Compared with the traditional batch reactor, the microreactor showed several advantages, including less reaction time, milder reaction temperature, higher yield and selectivity for 1,5-ditosyl-1,5-diazocane-3,7-dione. Moreover, the microreactor could ensure the safer and large-scale industrial production of 1,5-ditosyl-1,5-diazocane-3,7-dione. However, some solids produced in Swern oxidation which might block the channels (diameter of 0.3 mm) in the microreactor. To overcome the challenges, the experimental device was modified to suit for Swern oxidation, contributing to wider application of the microreactor. Besides, response surface methodology (RSM) was introduced and an appropriate mathematical model was built to optimize experimental conditions. The optimum experimental parameters were recommended as 7.8 ℃ for the reaction temperature, 7.7 mL/min for the flow rate, and 6% for the concentration of oxalyl chloride. The actual yield of 1,5-ditosyl-1,5-diazocane-3,7-dione was 89.7%, which was in great agreement with the highest predicted yield (90.1%).
中文翻译:

使用响应面法合成 1,5-ditosyl-1,5-diazocane-3,7-dione 的微流体连续流合成
摘要 3,3,7,7-四(二氟氨基)八氢-1,5-二硝基-1,5-重氮辛 (HNFX) 是一种具有四个二氟氨基 (-NF2) 的高密度高能氧化剂。在这项研究中,1,5-ditosyl-1,5-diazocane-3,7-dione 作为合成 HNFX 的重要中间体,分别在传统的间歇式反应器和连续流动微反应器中使用 Swern 氧化成功合成。1,5-dotosyl-1,5-diazocane-3,7-dione 通过 HPLC、FTIR、1H NMR、13C NMR、质谱和 X 射线晶体衍射表征。与传统的间歇式反应器相比,该微反应器具有反应时间短、反应温度温和、产率和对 1,5-ditosyl-1,5-diazocane-3,7-dione 具有更高的选择性等优点。此外,微反应器可以确保1,5-ditosyl-1的更安全和大规模的工业化生产,5-diazocane-3,7-dione。然而,在 Swern 氧化中产生的一些固体可能会堵塞微反应器中的通道(直径为 0.3 毫米)。为了克服这些挑战,对实验装置进行了修改以适应 Swern 氧化,有助于微反应器的更广泛应用。此外,还引入了响应面法(RSM),并建立了合适的数学模型来优化实验条件。推荐最佳实验参数为反应温度7.8 ℃,流速7.7 mL/min,草酰氯浓度6%。1,5-ditosyl-1,5-diazocane-3,7-dione 的实际产率为 89.7%,与预测的最高产率(90.1%)非常吻合。3 毫米)在微反应器中。为了克服这些挑战,对实验装置进行了修改以适应 Swern 氧化,有助于微反应器的更广泛应用。此外,还引入了响应面法(RSM),并建立了合适的数学模型来优化实验条件。推荐最佳实验参数为反应温度7.8 ℃,流速7.7 mL/min,草酰氯浓度6%。1,5-ditosyl-1,5-diazocane-3,7-dione 的实际产率为 89.7%,与预测的最高产率(90.1%)非常吻合。3 毫米)在微反应器中。为了克服这些挑战,对实验装置进行了修改以适应 Swern 氧化,有助于微反应器的更广泛应用。此外,还引入了响应面法(RSM),并建立了合适的数学模型来优化实验条件。推荐最佳实验参数为反应温度7.8 ℃,流速7.7 mL/min,草酰氯浓度6%。1,5-ditosyl-1,5-diazocane-3,7-dione 的实际产率为 89.7%,与预测的最高产率(90.1%)非常吻合。引入响应面方法 (RSM) 并建立适当的数学模型来优化实验条件。推荐最佳实验参数为反应温度7.8 ℃,流速7.7 mL/min,草酰氯浓度6%。1,5-ditosyl-1,5-diazocane-3,7-dione 的实际产率为 89.7%,与预测的最高产率(90.1%)非常吻合。引入响应面方法 (RSM) 并建立适当的数学模型来优化实验条件。推荐最佳实验参数为反应温度7.8 ℃,流速7.7 mL/min,草酰氯浓度6%。1,5-ditosyl-1,5-diazocane-3,7-dione 的实际产率为 89.7%,与预测的最高产率(90.1%)非常吻合。
更新日期:2020-02-01
中文翻译:

使用响应面法合成 1,5-ditosyl-1,5-diazocane-3,7-dione 的微流体连续流合成
摘要 3,3,7,7-四(二氟氨基)八氢-1,5-二硝基-1,5-重氮辛 (HNFX) 是一种具有四个二氟氨基 (-NF2) 的高密度高能氧化剂。在这项研究中,1,5-ditosyl-1,5-diazocane-3,7-dione 作为合成 HNFX 的重要中间体,分别在传统的间歇式反应器和连续流动微反应器中使用 Swern 氧化成功合成。1,5-dotosyl-1,5-diazocane-3,7-dione 通过 HPLC、FTIR、1H NMR、13C NMR、质谱和 X 射线晶体衍射表征。与传统的间歇式反应器相比,该微反应器具有反应时间短、反应温度温和、产率和对 1,5-ditosyl-1,5-diazocane-3,7-dione 具有更高的选择性等优点。此外,微反应器可以确保1,5-ditosyl-1的更安全和大规模的工业化生产,5-diazocane-3,7-dione。然而,在 Swern 氧化中产生的一些固体可能会堵塞微反应器中的通道(直径为 0.3 毫米)。为了克服这些挑战,对实验装置进行了修改以适应 Swern 氧化,有助于微反应器的更广泛应用。此外,还引入了响应面法(RSM),并建立了合适的数学模型来优化实验条件。推荐最佳实验参数为反应温度7.8 ℃,流速7.7 mL/min,草酰氯浓度6%。1,5-ditosyl-1,5-diazocane-3,7-dione 的实际产率为 89.7%,与预测的最高产率(90.1%)非常吻合。3 毫米)在微反应器中。为了克服这些挑战,对实验装置进行了修改以适应 Swern 氧化,有助于微反应器的更广泛应用。此外,还引入了响应面法(RSM),并建立了合适的数学模型来优化实验条件。推荐最佳实验参数为反应温度7.8 ℃,流速7.7 mL/min,草酰氯浓度6%。1,5-ditosyl-1,5-diazocane-3,7-dione 的实际产率为 89.7%,与预测的最高产率(90.1%)非常吻合。3 毫米)在微反应器中。为了克服这些挑战,对实验装置进行了修改以适应 Swern 氧化,有助于微反应器的更广泛应用。此外,还引入了响应面法(RSM),并建立了合适的数学模型来优化实验条件。推荐最佳实验参数为反应温度7.8 ℃,流速7.7 mL/min,草酰氯浓度6%。1,5-ditosyl-1,5-diazocane-3,7-dione 的实际产率为 89.7%,与预测的最高产率(90.1%)非常吻合。引入响应面方法 (RSM) 并建立适当的数学模型来优化实验条件。推荐最佳实验参数为反应温度7.8 ℃,流速7.7 mL/min,草酰氯浓度6%。1,5-ditosyl-1,5-diazocane-3,7-dione 的实际产率为 89.7%,与预测的最高产率(90.1%)非常吻合。引入响应面方法 (RSM) 并建立适当的数学模型来优化实验条件。推荐最佳实验参数为反应温度7.8 ℃,流速7.7 mL/min,草酰氯浓度6%。1,5-ditosyl-1,5-diazocane-3,7-dione 的实际产率为 89.7%,与预测的最高产率(90.1%)非常吻合。































 京公网安备 11010802027423号
京公网安备 11010802027423号