当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure of ribosome-bound azole-modified peptide phazolicin rationalizes its species-specific mode of bacterial translation inhibition.
Nature Communications ( IF 14.7 ) Pub Date : 2019-10-08 , DOI: 10.1038/s41467-019-12589-5 Dmitrii Y Travin 1, 2 , Zoe L Watson 3 , Mikhail Metelev 1, 2, 4 , Fred R Ward 5 , Ilya A Osterman 1, 6 , Irina M Khven 6, 7 , Nelli F Khabibullina 8, 9 , Marina Serebryakova 10 , Peter Mergaert 11 , Yury S Polikanov 8, 12 , Jamie H D Cate 3, 5 , Konstantin Severinov 1, 2, 13
Nature Communications ( IF 14.7 ) Pub Date : 2019-10-08 , DOI: 10.1038/s41467-019-12589-5 Dmitrii Y Travin 1, 2 , Zoe L Watson 3 , Mikhail Metelev 1, 2, 4 , Fred R Ward 5 , Ilya A Osterman 1, 6 , Irina M Khven 6, 7 , Nelli F Khabibullina 8, 9 , Marina Serebryakova 10 , Peter Mergaert 11 , Yury S Polikanov 8, 12 , Jamie H D Cate 3, 5 , Konstantin Severinov 1, 2, 13
Affiliation
|
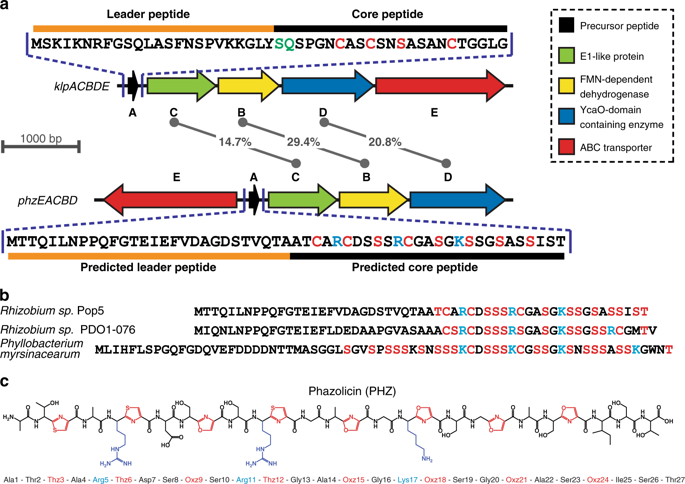
Ribosome-synthesized post-translationally modified peptides (RiPPs) represent a rapidly expanding class of natural products with various biological activities. Linear azol(in)e-containing peptides (LAPs) comprise a subclass of RiPPs that display outstanding diversity of mechanisms of action while sharing common structural features. Here, we report the discovery of a new LAP biosynthetic gene cluster in the genome of Rhizobium Pop5, which encodes the precursor peptide and modification machinery of phazolicin (PHZ) - an extensively modified peptide exhibiting narrow-spectrum antibacterial activity against some symbiotic bacteria of leguminous plants. The cryo-EM structure of the Escherichia coli 70S-PHZ complex reveals that the drug interacts with the 23S rRNA and uL4/uL22 proteins and obstructs ribosomal exit tunnel in a way that is distinct from other compounds. We show that the uL4 loop sequence determines the species-specificity of antibiotic action. PHZ expands the known diversity of LAPs and may be used in the future as biocontrol agent for agricultural needs.
中文翻译:

核糖体结合的唑修饰的肽phazolicin的结构使其细菌翻译抑制的物种特异性模式合理化。
核糖体合成的翻译后修饰肽(RiPPs)代表了具有各种生物活性的天然产物的快速扩展类别。线性的含氮杂(in)e的肽(LAP)包含RiPPs的一个子类,该子类显示出出色的作用机理多样性,同时具有共同的结构特征。在这里,我们报告在根瘤菌Pop5基因组中发现一个新的LAP生物合成基因簇,该簇编码前体肽和phazolicin(PHZ)的修饰机制-广泛修饰的肽对豆类的一些共生细菌表现出窄谱抗菌活性植物。大肠杆菌70S-PHZ复合物的冷冻EM结构表明,该药物与23S rRNA和uL4 / uL22蛋白相互作用,并以不同于其他化合物的方式阻塞了核糖体出口通道。我们表明,uL4环序列决定了抗生素作用的物种特异性。PHZ扩大了LAP的已知多样性,将来可以用作农业需求的生物防治剂。
更新日期:2019-10-08
中文翻译:

核糖体结合的唑修饰的肽phazolicin的结构使其细菌翻译抑制的物种特异性模式合理化。
核糖体合成的翻译后修饰肽(RiPPs)代表了具有各种生物活性的天然产物的快速扩展类别。线性的含氮杂(in)e的肽(LAP)包含RiPPs的一个子类,该子类显示出出色的作用机理多样性,同时具有共同的结构特征。在这里,我们报告在根瘤菌Pop5基因组中发现一个新的LAP生物合成基因簇,该簇编码前体肽和phazolicin(PHZ)的修饰机制-广泛修饰的肽对豆类的一些共生细菌表现出窄谱抗菌活性植物。大肠杆菌70S-PHZ复合物的冷冻EM结构表明,该药物与23S rRNA和uL4 / uL22蛋白相互作用,并以不同于其他化合物的方式阻塞了核糖体出口通道。我们表明,uL4环序列决定了抗生素作用的物种特异性。PHZ扩大了LAP的已知多样性,将来可以用作农业需求的生物防治剂。































 京公网安备 11010802027423号
京公网安备 11010802027423号