当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Heme Propionate Staples the Structure of Cytochrome c for Methionine Ligation to the Heme Iron.
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2019-10-07 , DOI: 10.1021/acs.inorgchem.9b02111 Yunling Deng 1 , Madeline L Weaver 2 , Kevin R Hoke 2 , Ekaterina V Pletneva 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2019-10-07 , DOI: 10.1021/acs.inorgchem.9b02111 Yunling Deng 1 , Madeline L Weaver 2 , Kevin R Hoke 2 , Ekaterina V Pletneva 1
Affiliation
|
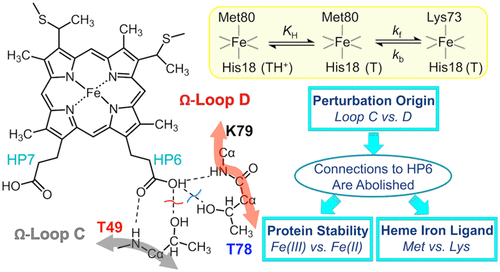
Ligand-switch reactions at the heme iron are common in biological systems, but their mechanisms and the features of the polypeptide fold that support dual ligation are not well understood. In cytochrome c (cyt c), two low-stability loops (Ω-loop C and Ω-loop D) are connected by the heme propionate HP6. At alkaline pH, the native Met80 ligand from Ω-loop D switches to a Lys residue from the same loop. Deprotonation of an as yet unknown group triggers the alkaline transition. We have created the two cyt c variants T49V/K79G and T78V/K79G with altered connections of these two loops to HP6. Electronic absorption, NMR, and EPR studies demonstrate that at pH 7.4 ferric forms of these variants are Lys-ligated, whereas ferrous forms maintain the native Met80 ligation. Measurements of protein stability, cyclic voltammetry, pH-jump and gated electron-transfer kinetics have revealed that these Thr to Val substitutions greatly affect the alkaline transition in both ferric and ferrous proteins. The substitutions modify the stability of the Met-ligated species and reduction potentials of the heme iron. The kinetics of ligand-switch processes are also altered, and analyses of these effects implicate redox-dependent differences in metal–ligand interactions and the role of the protein dynamics, including cross-talk between the two Ω-loops. With the two destabilized variants, it is possible to map energy levels for the Met- and Lys-ligated species in both ferric and ferrous proteins and assess the role of the protein scaffold in redox-dependent preferences for these two ligands. The estimated shift in the heme iron reduction potential upon deprotonation of the “trigger” group is consistent with those associated with deprotonation of an HP, suggesting that HP6, on its own or as a part of a hydrogen-bonded cluster, is a likely “trigger” for the Met to Lys ligand switch.
中文翻译:

血红素丙酸酯修饰细胞色素c的结构,用于蛋氨酸与血红素铁的连接。
血红素铁上的配体转换反应在生物系统中很常见,但它们对支持双重连接的机制和多肽折叠特征尚不十分了解。在细胞色素c(cyt c)中,两个低稳定性环(Ω环C和Ω环D)通过丙酸血红素HP6连接。在碱性pH值下,来自Ω环D的天然Met80配体从同一环转换为Lys残基。尚不清楚的基团的去质子化触发了碱性转变。我们创建了两个cyt c变体T49V / K79G和T78V / K79G,这两个回路与HP6的连接已更改。电子吸收,NMR和EPR研究表明,在pH 7.4时,这些变体的铁形式被Lys连接,而亚铁形式则保持了天然的Met80连接。对蛋白质稳定性,循环伏安法,pH跳跃和门控电子转移动力学的测量表明,这些从Thr到Val的取代极大地影响了铁蛋白和亚铁蛋白的碱性转变。取代改变了Met连接的物种的稳定性和血红素铁的还原电位。配体转换过程的动力学也发生了变化,对这些效应的分析暗示了氧化还原依赖性金属-配体相互作用的差异以及蛋白质动力学的作用,包括两个Ω环之间的串扰。使用这两个不稳定的变体,可以绘制铁蛋白和亚铁蛋白中Met和Lys连接的物种的能级图,并评估蛋白支架在这两个配体的氧化还原依赖性偏好中的作用。“触发”基团去质子化后,血红素还原电位的估计变化与HP质子化相关的变化相一致,表明HP6本身或作为氢键簇的一部分可能是“ Met到Lys配体转换的“触发”。
更新日期:2019-10-08
中文翻译:

血红素丙酸酯修饰细胞色素c的结构,用于蛋氨酸与血红素铁的连接。
血红素铁上的配体转换反应在生物系统中很常见,但它们对支持双重连接的机制和多肽折叠特征尚不十分了解。在细胞色素c(cyt c)中,两个低稳定性环(Ω环C和Ω环D)通过丙酸血红素HP6连接。在碱性pH值下,来自Ω环D的天然Met80配体从同一环转换为Lys残基。尚不清楚的基团的去质子化触发了碱性转变。我们创建了两个cyt c变体T49V / K79G和T78V / K79G,这两个回路与HP6的连接已更改。电子吸收,NMR和EPR研究表明,在pH 7.4时,这些变体的铁形式被Lys连接,而亚铁形式则保持了天然的Met80连接。对蛋白质稳定性,循环伏安法,pH跳跃和门控电子转移动力学的测量表明,这些从Thr到Val的取代极大地影响了铁蛋白和亚铁蛋白的碱性转变。取代改变了Met连接的物种的稳定性和血红素铁的还原电位。配体转换过程的动力学也发生了变化,对这些效应的分析暗示了氧化还原依赖性金属-配体相互作用的差异以及蛋白质动力学的作用,包括两个Ω环之间的串扰。使用这两个不稳定的变体,可以绘制铁蛋白和亚铁蛋白中Met和Lys连接的物种的能级图,并评估蛋白支架在这两个配体的氧化还原依赖性偏好中的作用。“触发”基团去质子化后,血红素还原电位的估计变化与HP质子化相关的变化相一致,表明HP6本身或作为氢键簇的一部分可能是“ Met到Lys配体转换的“触发”。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号