当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Oxidation of VOCs over SmMnO3 Perovskites: Catalyst Synthesis, Change Mechanism of Active Species, and Degradation Path of Toluene
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2019-10-07 , DOI: 10.1021/acs.inorgchem.9b02518 Lizhong Liu 1 , Jiangtian Sun 2 , Jiandong Ding 1 , Yan Zhang 1 , Jinping Jia 3 , Tonghua Sun 3
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2019-10-07 , DOI: 10.1021/acs.inorgchem.9b02518 Lizhong Liu 1 , Jiangtian Sun 2 , Jiandong Ding 1 , Yan Zhang 1 , Jinping Jia 3 , Tonghua Sun 3
Affiliation
|
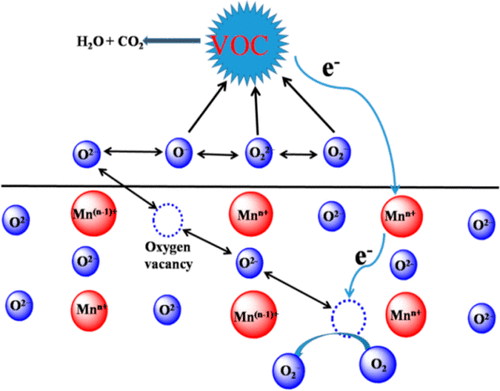
Highly active samarium manganese perovskite oxides were successfully prepared by employing self-molten-polymerization, coprecipitation, sol–gel, and impregnation methods. The physicochemical properties of perovskite oxides were investigated by XRD, N2 adsorption–desorption, XPS, and H2-TPR. Their catalytic performances were compared via the catalytic oxidation of toluene. The perovskite prepared by self-molten-polymerization possessed the highest catalytic capacity, which can be ascribed to its higher oxygen adspecies concentration (Olatt/Oads = 0.53), higher surface Mn4+/Mn3+ ratio (Mn4+/Mn3+ = 0.95), and best low-temperature reducibility (H2 consumption = 0.27; below 350 °C). The most active catalyst also exhibited good cycling and long-term stability for toluene oxidation. After a multistep cycle reaction and a long-term reaction of 42 h, the toluene conversion maintained above 99.9% at 270 °C. Mechanistic study hinted that lattice oxygen was involved in toluene oxidation. The oxidation reaction was dependent on the synergism of lattice oxygen, adsorbed oxygen, and oxygen vacancies. The degradation pathway of toluene, researched by diffuse reflectance infrared Fourier transform spectroscopy and online mass spectrometry technologies, demonstrated that a series of organic byproducts existed at a relatively low temperature. This work provides an efficient and practical method for selecting highly active catalysts and for exploring the catalytic mechanism for the removal of atmospheric environmental pollution.
中文翻译:

SmMnO 3钙钛矿上VOC的催化氧化:催化剂的合成,活性物种的变化机理和甲苯的降解途径
高活性sa钙钛矿锰氧化物是通过采用自熔聚合,共沉淀,溶胶-凝胶和浸渍方法成功制备的。通过XRD,N 2吸附-解吸,XPS和H 2 -TPR研究了钙钛矿氧化物的物理化学性质。通过甲苯的催化氧化比较了它们的催化性能。通过自熔融聚合制备的钙钛矿所具有的最高的催化能力,这可以归因于它的更高的氧浓度adspecies(O LATT / O广告= 0.53),面更高的Mn 4+ / Mn为3+比(锰4+ /锰3+= 0.95)和最佳的低温还原性(H 2消耗= 0.27; 低于350°C)。最具活性的催化剂还表现出良好的循环性和对甲苯氧化的长期稳定性。经过多步循环反应和42 h的长期反应后,甲苯的转化率在270°C时保持在99.9%以上。机理研究表明,晶格氧参与了甲苯的氧化。氧化反应取决于晶格氧,吸附氧和氧空位的协同作用。通过漫反射红外傅里叶变换光谱法和在线质谱技术研究了甲苯的降解途径,结果表明一系列有机副产物在相对较低的温度下存在。
更新日期:2019-10-08
中文翻译:

SmMnO 3钙钛矿上VOC的催化氧化:催化剂的合成,活性物种的变化机理和甲苯的降解途径
高活性sa钙钛矿锰氧化物是通过采用自熔聚合,共沉淀,溶胶-凝胶和浸渍方法成功制备的。通过XRD,N 2吸附-解吸,XPS和H 2 -TPR研究了钙钛矿氧化物的物理化学性质。通过甲苯的催化氧化比较了它们的催化性能。通过自熔融聚合制备的钙钛矿所具有的最高的催化能力,这可以归因于它的更高的氧浓度adspecies(O LATT / O广告= 0.53),面更高的Mn 4+ / Mn为3+比(锰4+ /锰3+= 0.95)和最佳的低温还原性(H 2消耗= 0.27; 低于350°C)。最具活性的催化剂还表现出良好的循环性和对甲苯氧化的长期稳定性。经过多步循环反应和42 h的长期反应后,甲苯的转化率在270°C时保持在99.9%以上。机理研究表明,晶格氧参与了甲苯的氧化。氧化反应取决于晶格氧,吸附氧和氧空位的协同作用。通过漫反射红外傅里叶变换光谱法和在线质谱技术研究了甲苯的降解途径,结果表明一系列有机副产物在相对较低的温度下存在。































 京公网安备 11010802027423号
京公网安备 11010802027423号