当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Facile fabrication of a hierarchical NiCoFeP hollow nanoprism for efficient oxygen evolution in the Zn–air battery†
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2019-10-08 , DOI: 10.1039/c9ta09239k Bin He 1, 2, 3, 4, 5 , Chunyu Xu 5, 6, 7, 8, 9 , Yawen Tang 5, 6, 7, 8, 9 , Yong Qian 5, 6, 7, 8, 9 , Hongke Liu 5, 6, 7, 8, 9 , Qingli Hao 1, 2, 3, 4, 5 , Zhi Su 5, 6, 7, 8, 9
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2019-10-08 , DOI: 10.1039/c9ta09239k Bin He 1, 2, 3, 4, 5 , Chunyu Xu 5, 6, 7, 8, 9 , Yawen Tang 5, 6, 7, 8, 9 , Yong Qian 5, 6, 7, 8, 9 , Hongke Liu 5, 6, 7, 8, 9 , Qingli Hao 1, 2, 3, 4, 5 , Zhi Su 5, 6, 7, 8, 9
Affiliation
|
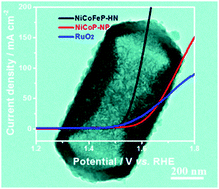
The rational construction of the chemical composition and morphological structure of electrocatalysts has been considered as the key to their electrochemical performance and energy storage. Herein, we report the facile fabrication of the hierarchical Ni2P–Co2P–Fe2P hybrid hollow nanoprism (abbreviated as NiCoFeP-HN), which could efficiently work as the oxygen evolution reaction (OER) electrocatalyst in the rechargeable Zn–air battery. NiCoFeP-HN was synthesized via an in situ ion exchange reaction of nickel–cobalt precursors with [Fe(CN)6]3− at room temperature and the subsequent phosphorization at 300 °C under N2. The ion exchange procedure was crucial for preserving the nanoprism morphology and enhancing the OER performance. NiCoFeP-HN exhibited satisfactory activity with a small overpotential of 294 mV at 10 mA cm−2 and remarkable stability for over 12 h. Compared to the conventional RuO2 + Pt/C Zn–air battery, the NiCoFeP-HN + Pt/C based Zn–air battery has exhibited superior energy density and much better cycling stability. This work presents a facile and efficient avenue for the construction of hollow nanostructures and hybrid compositions, and provides a promising candidate with good electrochemical performance and energy storage.
中文翻译:

便于制造分层NiCoFeP空心纳米棱镜,以在锌空气电池中有效释放氧气†
电催化剂化学成分和形态结构的合理构建被认为是其电化学性能和储能的关键。本文中,我们报道了Ni 2 P–Co 2 P–Fe 2 P杂化空心纳米棱镜(缩写为NiCoFeP-HN)的简便制备方法,该方法可以有效地用作可再充电Zn–中的氧释放反应(OER)电催化剂。空气电池。NiCoFeP-HN合成通过一个在原位用的[Fe(CN)的镍-钴前体的离子交换反应6 ] 3-在室温和在300℃氮气氛下随后的磷化2。离子交换程序对于保持纳米棱镜形态和增强OER性能至关重要。NiCoFeP-HN表现出令人满意的活性,在10 mA cm -2时具有294 mV的小过电位,并且在超过12 h内具有出色的稳定性。与传统的RuO 2 + Pt / C Zn-空气电池相比,基于NiCoFeP-HN + Pt / C的Zn-空气电池具有更高的能量密度和更好的循环稳定性。这项工作为中空纳米结构和杂化组合物的构建提供了简便有效的途径,并提供了具有良好电化学性能和能量存储的有前途的候选对象。
更新日期:2019-11-05
中文翻译:

便于制造分层NiCoFeP空心纳米棱镜,以在锌空气电池中有效释放氧气†
电催化剂化学成分和形态结构的合理构建被认为是其电化学性能和储能的关键。本文中,我们报道了Ni 2 P–Co 2 P–Fe 2 P杂化空心纳米棱镜(缩写为NiCoFeP-HN)的简便制备方法,该方法可以有效地用作可再充电Zn–中的氧释放反应(OER)电催化剂。空气电池。NiCoFeP-HN合成通过一个在原位用的[Fe(CN)的镍-钴前体的离子交换反应6 ] 3-在室温和在300℃氮气氛下随后的磷化2。离子交换程序对于保持纳米棱镜形态和增强OER性能至关重要。NiCoFeP-HN表现出令人满意的活性,在10 mA cm -2时具有294 mV的小过电位,并且在超过12 h内具有出色的稳定性。与传统的RuO 2 + Pt / C Zn-空气电池相比,基于NiCoFeP-HN + Pt / C的Zn-空气电池具有更高的能量密度和更好的循环稳定性。这项工作为中空纳米结构和杂化组合物的构建提供了简便有效的途径,并提供了具有良好电化学性能和能量存储的有前途的候选对象。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号