Cell Research ( IF 28.1 ) Pub Date : 2019-10-07 , DOI: 10.1038/s41422-019-0234-8 Xianfa Yang 1 , Boqiang Hu 2 , Jiaoyang Liao 1 , Yunbo Qiao 3 , Yingying Chen 1 , Yun Qian 1 , Su Feng 1 , Fang Yu 1 , Ji Dong 2 , Yu Hou 2 , He Xu 1 , Ran Wang 1 , Guangdun Peng 1, 4, 5, 6 , Jinsong Li 1, 7 , Fuchou Tang 2, 8, 9 , Naihe Jing 1, 6, 7
|
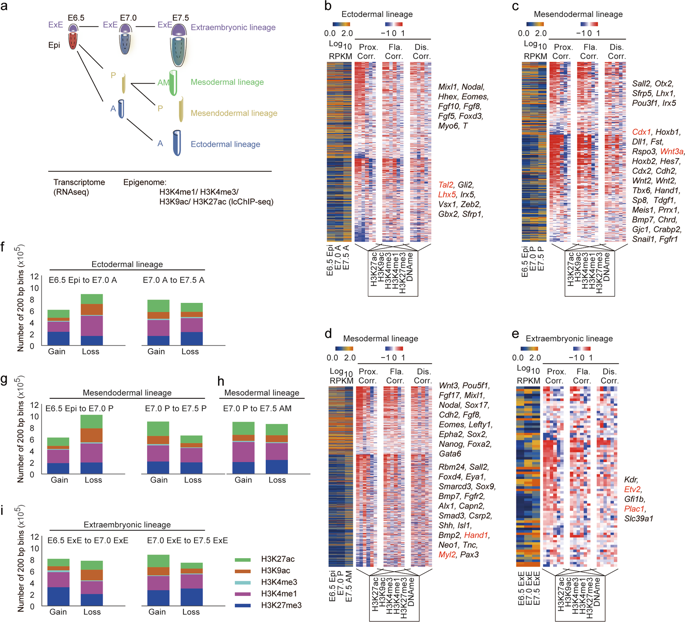
Primary germ layers have the potential to form all tissues in the mature organism, and their formation during gastrulation requires precise epigenetic modulation of both proximal and distal regulatory elements. Previous studies indicated that spatial and temporal patterns of gene expression in the gastrula predispose individual regions to distinct cell fates. However, the underlying epigenetic mechanisms remain largely unexplored. Here, we profile the spatiotemporal landscape of the epigenome and transcriptome of the mouse gastrula. We reveal the asynchronous dynamics of proximal chromatin states during germ layer formation as well as unique gastrula-specific epigenomic features of regulatory elements, which have strong usage turnover dynamics and clear germ layer-specific signatures. Importantly, we also find that enhancers around organogenetic genes, which are weakly expressed at the gastrulation stage, are frequently pre-marked by histone H3 lysine 27 acetylation (H3K27ac) in the gastrula. By using the transgenic mice and genome editing system, we demonstrate that a pre-marked enhancer, which is located in the intron of a brain-specific gene 2510009E07Rik, exhibits specific enhancer activity in the ectoderm and future brain tissue, and also executes important function during mouse neural differentiation. Taken together, our study provides the comprehensive epigenetic information for embryonic patterning during mouse gastrulation, demonstrates the importance of gastrula pre-marked enhancers in regulating the correct development of the mouse embryo, and thus broadens the current understanding of mammalian embryonic development and related diseases.
中文翻译:

小鼠原肠胚中不同的增强子特征描绘了胚胎发育过程中进行性细胞命运的连续性。
初级胚层有可能在成熟有机体中形成所有组织,它们在原肠胚形成过程中的形成需要对近端和远端调节元件进行精确的表观遗传调节。以前的研究表明,原肠胚中基因表达的空间和时间模式使各个区域倾向于不同的细胞命运。然而,潜在的表观遗传机制在很大程度上仍未被探索。在这里,我们描述了小鼠原肠胚的表观基因组和转录组的时空景观。我们揭示了胚层形成过程中近端染色质状态的异步动态以及调节元件独特的原肠胚特异性表观基因组特征,这些元件具有强大的使用周转动态和清晰的胚层特异性特征。重要的,我们还发现,在原肠胚形成阶段弱表达的器官发生基因周围的增强子经常被原肠胚中的组蛋白 H3 赖氨酸 27 乙酰化 (H3K27ac) 预先标记。通过使用转基因小鼠和基因组编辑系统,我们证明了一种预先标记的增强子,它位于大脑特异性基因的内含子中2510009E07Rik,在外胚层和未来的脑组织中表现出特定的增强子活性,还在小鼠神经分化过程中发挥重要作用。总之,我们的研究为小鼠原肠胚形成过程中的胚胎模式提供了全面的表观遗传信息,证明了原肠胚预先标记的增强子在调节小鼠胚胎正确发育中的重要性,从而拓宽了目前对哺乳动物胚胎发育和相关疾病的理解。






























 京公网安备 11010802027423号
京公网安备 11010802027423号