当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multifunctional dual-mesoporous silica nanoparticles loaded with a protein and dual antitumor drugs as a targeted delivery system†
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2019-10-04 , DOI: 10.1039/c9nj03001h Fangxiang Song 1, 2, 3, 4 , Yan Li 2, 3, 4, 5 , Shuai Wang 2, 3, 4, 5 , Li Zhang 1, 2, 3, 4 , QianLin Chen 1, 2, 3, 4
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2019-10-04 , DOI: 10.1039/c9nj03001h Fangxiang Song 1, 2, 3, 4 , Yan Li 2, 3, 4, 5 , Shuai Wang 2, 3, 4, 5 , Li Zhang 1, 2, 3, 4 , QianLin Chen 1, 2, 3, 4
Affiliation
|
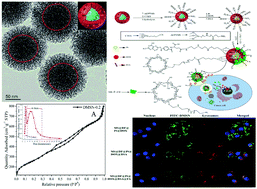
Herein, dual-mesoporous structure silica (with pore sizes from 2 to 4 nm and from 4 to 16 nm) simultaneously modified with amino and carboxyl groups was successfully synthesized. Doxorubicin (DOX) and cisplatin (Pt) were loaded onto the interior of the spherical mesoporous silica carrier material, and the macromolecular model drug bovine serum albumin (BSA) was loaded onto the outer layer using aminoguanidine-cyclodextrin as a blocking agent; moreover, folic acid (FA) was introduced to achieve targeted functionalization. Thus, a multi-drug and protein therapy system was constructed with a targeting functionality and pH stimulation-responsive controlled release system. Drug-loaded testing results showed that the amino and carboxyl-functionalized nanoparticles displayed high degrees of drug loading for DOX, Pt and BSA. The loading rate of BSA reached 33%. In vitro drug release experiments confirmed that this drug carrier system could achieve “zero pre-release”. The cytotoxicity of the dual-drug system was significantly higher than those of the single-load drug delivery systems although it decreased the toxicity of the drug itself towards the HeLa cells. The multi-drug and protein therapy system with targeting functionality showed better synergistic targeted therapeutic effects for HeLa cells. All the experiments show that the combination of multi-drugs and protein is a promising strategy.
中文翻译:

装有蛋白质和抗肿瘤双重药物的多功能双中孔二氧化硅纳米颗粒,作为靶向递送系统†
在此,成功地合成了同时用氨基和羧基改性的双介孔结构二氧化硅(孔径为2至4 nm和4至16 nm)。将阿霉素(DOX)和顺铂(Pt)装载到球形介孔二氧化硅载体材料的内部,并使用氨基胍-环糊精作为封闭剂将大分子模型药物牛血清白蛋白(BSA)装载到外层;此外,引入叶酸(FA)以实现目标功能化。因此,构建了具有靶向功能和pH刺激响应型控释系统的多药物和蛋白质治疗系统。载药测试结果表明,氨基和羧基官能化的纳米颗粒对DOX,Pt和BSA表现出很高的载药量。体外药物释放实验证实,这种药物载体系统可以实现“零预释放”。尽管双药系统降低了药物本身对HeLa细胞的毒性,但它的细胞毒性明显高于单负荷给药系统。具有靶向功能的多药物和蛋白质治疗系统对HeLa细胞显示出更好的协同靶向治疗效果。所有的实验表明,多种药物和蛋白质的结合是一种有前途的策略。
更新日期:2019-11-13
中文翻译:

装有蛋白质和抗肿瘤双重药物的多功能双中孔二氧化硅纳米颗粒,作为靶向递送系统†
在此,成功地合成了同时用氨基和羧基改性的双介孔结构二氧化硅(孔径为2至4 nm和4至16 nm)。将阿霉素(DOX)和顺铂(Pt)装载到球形介孔二氧化硅载体材料的内部,并使用氨基胍-环糊精作为封闭剂将大分子模型药物牛血清白蛋白(BSA)装载到外层;此外,引入叶酸(FA)以实现目标功能化。因此,构建了具有靶向功能和pH刺激响应型控释系统的多药物和蛋白质治疗系统。载药测试结果表明,氨基和羧基官能化的纳米颗粒对DOX,Pt和BSA表现出很高的载药量。体外药物释放实验证实,这种药物载体系统可以实现“零预释放”。尽管双药系统降低了药物本身对HeLa细胞的毒性,但它的细胞毒性明显高于单负荷给药系统。具有靶向功能的多药物和蛋白质治疗系统对HeLa细胞显示出更好的协同靶向治疗效果。所有的实验表明,多种药物和蛋白质的结合是一种有前途的策略。































 京公网安备 11010802027423号
京公网安备 11010802027423号