当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nucleobase-Functionalized 5-Aza-7-deazaguanine Ribo- and 2'-Deoxyribonucleosides: Glycosylation, Pd-Assisted Cross-Coupling, and Photophysical Properties.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-10-23 , DOI: 10.1021/acs.joc.9b01347 Peter Leonard 1 , Dasharath Kondhare 1 , Xenia Jentgens 1 , Constantin Daniliuc 2 , Frank Seela 1, 3
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-10-23 , DOI: 10.1021/acs.joc.9b01347 Peter Leonard 1 , Dasharath Kondhare 1 , Xenia Jentgens 1 , Constantin Daniliuc 2 , Frank Seela 1, 3
Affiliation
|
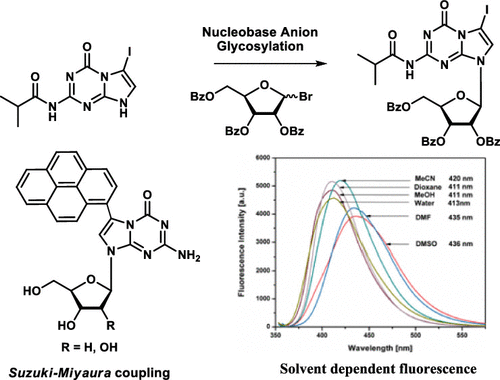
The special nucleobase recognition pattern of 5-aza-7-deazaguanine nucleosides makes them valuable for construction of homo purine DNA, silver-mediated base pairs, and expansion of the four letter genetic coding system. To widen the utility of 5-aza-7-deazaguanine nucleosides, side chains were introduced at position-7 of the nucleobase. As key compounds, 7-iodo nucleosides were synthesized. Nucleobase anion glycosylation of the iodo derivative of isobutyrylated 5-aza-7-deazaguanine with the bromo sugar of 2,3,5-tri-O-benzoyl-1-O-acetyl-d-ribofuranose gave the pure β-D anomeric N-9 glycosylation product (67%), whereas one-pot Vorbrüggen conditions gave only 42% of the iodinated nucleoside. The noniodinated nucleoside was formed in 84%. For the synthesis of 2'-deoxyribonucleosides, anion glycosylation performed with Hoffer's 2'-deoxyhalogenose yielded an anomeric mixture (α-D = 33% and β-D = 39%) of 2'-deoxyribonucleosides. Various side chain derivatives were prepared from nonprotected nucleosides by Pd-assisted Sonogashira or Suzuki-Miyaura cross-coupling. Among the functionalized ribonucleosides and anomeric 2'-deoxyribonucleosides, some of them showed strong fluorescence. Benzofuran and pyrene derivatives display high quantum yields in non-aqueous solvents and solvatochromism. Single-crystal X-ray analysis of 7-iodo-5-aza-7-deaza-2'-deoxyguanosine displayed intermolecular iodo-oxygen interactions in the crystal and channels filled with solvent molecules.
中文翻译:

核碱基功能化的5-Aza-7-脱氮鸟嘌呤核糖核苷和2'-脱氧核糖核苷:糖基化,Pd辅助交叉偶联和光物理性质。
5-氮杂-7-脱氮鸟嘌呤核苷的特殊核碱基识别模式使其对于构建高嘌呤DNA,银介导的碱基对以及扩展四字母遗传编码系统具有重要价值。为了扩大5-氮杂-7-脱氮鸟嘌呤核苷的效用,在核碱基的7位引入了侧链。作为关键化合物,合成了7碘核苷。异丁酰化的5-氮杂-7-脱氮鸟嘌呤的碘衍生物与2,3,5-三-O-苯甲酰基-1-O-乙酰基-d-呋喃核糖的溴糖的核碱基阴离子糖基化得到纯的β-D异头N -9糖基化产物(67%),而一锅Vorbrüggen条件仅产生42%的碘化核苷。非碘代核苷的形成率为84%。为了合成2'-脱氧核糖核苷,用Hoffer's 2'进行阴离子糖基化 -脱氧卤代糖产生2'-脱氧核糖核苷的异头混合物(α-D= 33%,β-D= 39%)。通过Pd辅助的Sonogashira或Suzuki-Miyaura交叉偶联,从未保护的核苷制备各种侧链衍生物。在功能化的核糖核苷和异头2'-脱氧核糖核苷中,其中一些显示强荧光。苯并呋喃和pyr衍生物在非水溶剂和溶剂变色中显示出高量子产率。7-iodo-5-aza-7-deaza-2'-deoxyguanosine的单晶X射线分析显示晶体和充满溶剂分子的通道中的分子间碘-氧相互作用。通过Pd辅助的Sonogashira或Suzuki-Miyaura交叉偶联,从未保护的核苷制备各种侧链衍生物。在功能化的核糖核苷和异头2'-脱氧核糖核苷中,其中一些显示强荧光。苯并呋喃和pyr衍生物在非水溶剂和溶剂变色中显示出高量子产率。7-iodo-5-aza-7-deaza-2'-deoxyguanosine的单晶X射线分析显示晶体和充满溶剂分子的通道中的分子间碘-氧相互作用。通过Pd辅助的Sonogashira或Suzuki-Miyaura交叉偶联,从未保护的核苷制备各种侧链衍生物。在功能化的核糖核苷和异头2'-脱氧核糖核苷中,其中一些显示强荧光。苯并呋喃和pyr衍生物在非水溶剂和溶剂变色中显示出高量子产率。7-iodo-5-aza-7-deaza-2'-deoxyguanosine的单晶X射线分析显示晶体和充满溶剂分子的通道中的分子间碘-氧相互作用。
更新日期:2019-10-24
中文翻译:

核碱基功能化的5-Aza-7-脱氮鸟嘌呤核糖核苷和2'-脱氧核糖核苷:糖基化,Pd辅助交叉偶联和光物理性质。
5-氮杂-7-脱氮鸟嘌呤核苷的特殊核碱基识别模式使其对于构建高嘌呤DNA,银介导的碱基对以及扩展四字母遗传编码系统具有重要价值。为了扩大5-氮杂-7-脱氮鸟嘌呤核苷的效用,在核碱基的7位引入了侧链。作为关键化合物,合成了7碘核苷。异丁酰化的5-氮杂-7-脱氮鸟嘌呤的碘衍生物与2,3,5-三-O-苯甲酰基-1-O-乙酰基-d-呋喃核糖的溴糖的核碱基阴离子糖基化得到纯的β-D异头N -9糖基化产物(67%),而一锅Vorbrüggen条件仅产生42%的碘化核苷。非碘代核苷的形成率为84%。为了合成2'-脱氧核糖核苷,用Hoffer's 2'进行阴离子糖基化 -脱氧卤代糖产生2'-脱氧核糖核苷的异头混合物(α-D= 33%,β-D= 39%)。通过Pd辅助的Sonogashira或Suzuki-Miyaura交叉偶联,从未保护的核苷制备各种侧链衍生物。在功能化的核糖核苷和异头2'-脱氧核糖核苷中,其中一些显示强荧光。苯并呋喃和pyr衍生物在非水溶剂和溶剂变色中显示出高量子产率。7-iodo-5-aza-7-deaza-2'-deoxyguanosine的单晶X射线分析显示晶体和充满溶剂分子的通道中的分子间碘-氧相互作用。通过Pd辅助的Sonogashira或Suzuki-Miyaura交叉偶联,从未保护的核苷制备各种侧链衍生物。在功能化的核糖核苷和异头2'-脱氧核糖核苷中,其中一些显示强荧光。苯并呋喃和pyr衍生物在非水溶剂和溶剂变色中显示出高量子产率。7-iodo-5-aza-7-deaza-2'-deoxyguanosine的单晶X射线分析显示晶体和充满溶剂分子的通道中的分子间碘-氧相互作用。通过Pd辅助的Sonogashira或Suzuki-Miyaura交叉偶联,从未保护的核苷制备各种侧链衍生物。在功能化的核糖核苷和异头2'-脱氧核糖核苷中,其中一些显示强荧光。苯并呋喃和pyr衍生物在非水溶剂和溶剂变色中显示出高量子产率。7-iodo-5-aza-7-deaza-2'-deoxyguanosine的单晶X射线分析显示晶体和充满溶剂分子的通道中的分子间碘-氧相互作用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号