当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Additional Thioflavin-T Binding Mode in Insulin Fibril Inner Core Region.
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2019-10-08 , DOI: 10.1021/acs.jpcb.9b08652 Mantas Ziaunys 1 , Vytautas Smirnovas 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2019-10-08 , DOI: 10.1021/acs.jpcb.9b08652 Mantas Ziaunys 1 , Vytautas Smirnovas 1
Affiliation
|
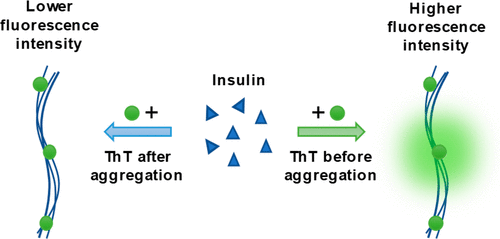
Amyloidogenic protein aggregation into fibrils is linked to several neurodegenerative disorders, such as Alzheimer's or Parkinson's disease. An amyloid specific fluorescent dye thioflavin-T (ThT) is often used to track the formation of these fibrils in vitro. Despite its wide application, it is still unknown how many types of ThT binding modes to amyloids exist, with multiple studies indicating varying numbers. In this work, we examine the binding of ThT to insulin fibrils generated at pH 2.4 and reveal a possible inner core binding mode which is not accessible to the dye molecule after aggregation occurs.
中文翻译:

胰岛素原纤维内核区的其他硫黄素-T结合模式。
淀粉样蛋白聚集成纤维与多种神经退行性疾病有关,例如阿尔茨海默氏病或帕金森氏病。淀粉样蛋白特异性荧光染料硫黄素-T(ThT)通常用于跟踪体外这些原纤维的形成。尽管有广泛的应用,但仍未知有多少种类型的ThT与淀粉样蛋白的结合模式,多项研究表明其数目不尽相同。在这项工作中,我们检查了ThT与在pH 2.4时产生的胰岛素原纤维的结合,并揭示了可能的内核结合模式,这种模式在聚集发生后是染料分子无法接近的。
更新日期:2019-10-08
中文翻译:

胰岛素原纤维内核区的其他硫黄素-T结合模式。
淀粉样蛋白聚集成纤维与多种神经退行性疾病有关,例如阿尔茨海默氏病或帕金森氏病。淀粉样蛋白特异性荧光染料硫黄素-T(ThT)通常用于跟踪体外这些原纤维的形成。尽管有广泛的应用,但仍未知有多少种类型的ThT与淀粉样蛋白的结合模式,多项研究表明其数目不尽相同。在这项工作中,我们检查了ThT与在pH 2.4时产生的胰岛素原纤维的结合,并揭示了可能的内核结合模式,这种模式在聚集发生后是染料分子无法接近的。






























 京公网安备 11010802027423号
京公网安备 11010802027423号