当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Application of E1cB Elimination in Asymmetric Organocatalytic Cascade Reactions To Construct Polyheterocyclic Compounds
Organic Letters ( IF 4.9 ) Pub Date : 2019-10-03 , DOI: 10.1021/acs.orglett.9b03138 Zhi-Hao You 1 , Ying-Han Chen 1 , Yu Tang 1, 2 , Yan-Kai Liu 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2019-10-03 , DOI: 10.1021/acs.orglett.9b03138 Zhi-Hao You 1 , Ying-Han Chen 1 , Yu Tang 1, 2 , Yan-Kai Liu 1, 2
Affiliation
|
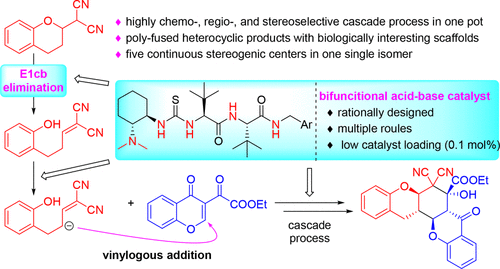
By introducing a carbon functionality at 2-position of chromane, the formal asymmetric functionalization of the 3-position of 2-substituted chromane has been realized via a highly chemo-, regio-, and stereoselective organocatalytic cascade reaction in a sequential one-pot manner involving an E1cB mechanism governed ring-opening process. Critical to our success was the design of a chiral dipeptide-based bifunctional acid–base organocatalyst, which exhibited high catalytic activity at low catalyst loading (1–0.1 mol %), leading to biologically interesting polyheterocyclic compounds.
中文翻译:

E1cB消除在不对称有机催化级联反应中构建多杂环化合物的应用
通过在色烷的2位上引入碳官能团,已通过高化学,区域和立体选择性有机催化级联反应以顺序一锅方式实现了2位取代的色烷3位的形式不对称官能化涉及E1cB机制控制的开环过程。我们成功的关键是设计基于手性二肽的双官能酸基有机催化剂,该催化剂在低催化剂负载量(1-0.1 mol%)下表现出高催化活性,从而导致了生物学上令人感兴趣的多杂环化合物。
更新日期:2019-10-03
中文翻译:

E1cB消除在不对称有机催化级联反应中构建多杂环化合物的应用
通过在色烷的2位上引入碳官能团,已通过高化学,区域和立体选择性有机催化级联反应以顺序一锅方式实现了2位取代的色烷3位的形式不对称官能化涉及E1cB机制控制的开环过程。我们成功的关键是设计基于手性二肽的双官能酸基有机催化剂,该催化剂在低催化剂负载量(1-0.1 mol%)下表现出高催化活性,从而导致了生物学上令人感兴趣的多杂环化合物。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号