Tetrahedron Letters ( IF 1.5 ) Pub Date : 2019-10-03 , DOI: 10.1016/j.tetlet.2019.151252 Noriyoshi Arai , Takeshi Ohkuma
|
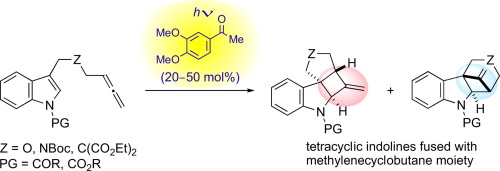
Irradiation of 3-(hexa-4,5-dienyl)indole derivatives in the presence of 3',4'-dimethoxyacetophenone by a high-pressure mercury lamp through Pyrex glass gave the corresponding [2+2] cycloaddition products stereoselectively in high yields. The major product was a methylenecyclobutane-fused angular tetracyclic spiroindoline derivative produced by the [2+2] cycloaddition through a parallel orientation. The minor product was a hexahydromethanocarbazole derivative through a crossed orientation. Electron-withdrawing substituents, such as acyl or alkoxycarbonyl, on the indole nitrogen were suitable for this reaction.
中文翻译:

丙二烯光敏性分子内[2 + 2]环加成反应立体选择性制备亚甲基环丁烷角四环螺吲哚啉。
在3',4'-二甲氧基苯乙酮的存在下,通过Pyrex玻璃用高压汞灯照射3-(六-4,5-二烯基)吲哚衍生物,以立体收率选择性地产生相应的[2 + 2]环加成产物。主要产物是由[2 + 2]环加成通过平行取向生成的亚甲基环丁烷稠合的角四环螺吲哚啉衍生物。次要产物是通过交叉取向的六氢甲氨基咔唑衍生物。吲哚氮上的吸电子取代基,例如酰基或烷氧基羰基适合于该反应。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号