当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Volcano Trend in Electrocatalytic CO2 Reduction Activity over Atomically Dispersed Metal Sites on Nitrogen-Doped Carbon
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-10-18 , DOI: 10.1021/acscatal.9b02594
Jingkun Li 1 , Paulina Pršlja 2 , Tatsuya Shinagawa , Antonio José Martín Fernández , Frank Krumeich , Kateryna Artyushkova 3 , Plamen Atanassov 4 , Andrea Zitolo 5 , Yecheng Zhou 2 , Rodrigo García-Muelas 2 , Núria López 2 , Javier Pérez-Ramírez , Frédéric Jaouen 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-10-18 , DOI: 10.1021/acscatal.9b02594
Jingkun Li 1 , Paulina Pršlja 2 , Tatsuya Shinagawa , Antonio José Martín Fernández , Frank Krumeich , Kateryna Artyushkova 3 , Plamen Atanassov 4 , Andrea Zitolo 5 , Yecheng Zhou 2 , Rodrigo García-Muelas 2 , Núria López 2 , Javier Pérez-Ramírez , Frédéric Jaouen 1
Affiliation
|
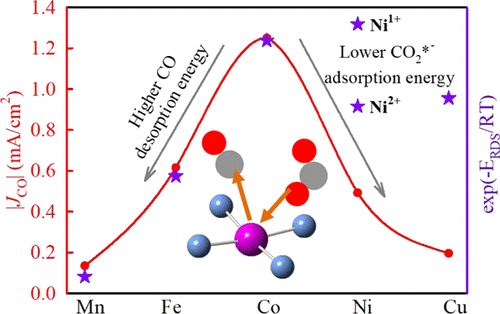
The development of catalysts for electrochemical reduction of carbon dioxide (eCO2RR) with high activity and selectivity remains a grand challenge to render the technology useable. As promising candidates, metal–nitrogen–carbon (MNC) catalysts with metal atoms present as atomically dispersed metal–Nx moieties (MNx, M = Mn, Fe, Co, Ni, and Cu) were investigated as model catalysts. The distinct activity for CO formation observed along the series of catalysts is attributed to the nature of the transition metal in MNx moieties because of otherwise similar composition, structure, and morphology of the carbon matrix. We identify a volcano trend between their activity toward CO formation and the nature of the transition metal in MNx sites, with Fe and/or Co at the top of the volcano, depending on the electrochemical potential. Regarding selectivity, FeNC, NiNC, and MnNC had Faradaic efficiency for CO >80%. To correctly model the active sites in operando conditions, experimental operando X-ray absorption near edge structure spectroscopy was performed to follow changes in the metal oxidation state with electrochemical potential. Co and Mn did not change the oxidation state with potential, whereas Fe and Ni were partially reduced and Cu largely reduced to Cu(0). Computational models then led to the identification of M2+N4–H2O as the most active centers in FeNC and CoNC, whereas Ni1+N4 was predicted as the most active one in NiNC at the considered potentials of −0.5 and −0.6 V versus the reversible hydrogen electrode. The experimental activity and selectivity could be rationalized from our density functional theory results, identifying in particular the difference between the binding energies for CO2*– and H* as a descriptor of selectivity toward CO. This in-depth understanding of the activity and selectivity based on the speciation of the metals for eCO2RR over atomically dispersed MNx sites provides guidelines for the rational design of MNC catalysts toward eCO2RR for their application in high-performance devices.
中文翻译:

氮掺杂碳上原子分散的金属位点上电催化CO 2还原活性的火山趋势
开发具有高活性和选择性的用于电化学还原二氧化碳的催化剂(eCO 2 RR)仍然是使该技术可用的巨大挑战。作为有前途的候选物,研究了以金属原子作为原子分散的金属N x部分(MN x,M = Mn,Fe,Co,Ni和Cu)存在的金属原子的金属-氮-碳(MNC)催化剂。沿一系列催化剂观察到的CO形成的独特活性归因于MN x部分中过渡金属的性质,因为碳基质的组成,结构和形态相似。我们确定了其对一氧化碳形成的活动与MN中过渡金属的性质之间的火山趋势。x个位置,根据电化学势,在火山顶部带有Fe和/或Co。关于选择性,FeNC,NiNC和MnNC在CO> 80%时具有法拉第效率。为了正确地模拟手术条件下的活性部位,进行了实验手术X射线吸收近边缘结构光谱法,以追踪金属氧化态随电化学势的变化。Co和Mn不会随电位改变氧化态,而Fe和Ni被部分还原,Cu大量还原为Cu(0)。然后,计算模型确定了M 2+ N 4 -H 2 O为FeNC和CoNC中最活跃的中心,而Ni 1+ N 4相对于可逆氢电极,在-0.5和-0.6 V的考虑电势下,NiCl被认为是NiNC中最活跃的一个。可以从我们的密度泛函理论结果中合理化实验活性和选择性,特别是确定CO 2 * -和H *的结合能之间的差异作为对CO的选择性的描述指标。这种对活性和选择性的深入理解基于金属在原子分散的MN x位置上的eCO 2 RR的形态,提供了MNC催化剂针对eCO 2 RR的合理设计的指导原则,以用于其在高性能设备中的应用。
更新日期:2019-10-19
中文翻译:

氮掺杂碳上原子分散的金属位点上电催化CO 2还原活性的火山趋势
开发具有高活性和选择性的用于电化学还原二氧化碳的催化剂(eCO 2 RR)仍然是使该技术可用的巨大挑战。作为有前途的候选物,研究了以金属原子作为原子分散的金属N x部分(MN x,M = Mn,Fe,Co,Ni和Cu)存在的金属原子的金属-氮-碳(MNC)催化剂。沿一系列催化剂观察到的CO形成的独特活性归因于MN x部分中过渡金属的性质,因为碳基质的组成,结构和形态相似。我们确定了其对一氧化碳形成的活动与MN中过渡金属的性质之间的火山趋势。x个位置,根据电化学势,在火山顶部带有Fe和/或Co。关于选择性,FeNC,NiNC和MnNC在CO> 80%时具有法拉第效率。为了正确地模拟手术条件下的活性部位,进行了实验手术X射线吸收近边缘结构光谱法,以追踪金属氧化态随电化学势的变化。Co和Mn不会随电位改变氧化态,而Fe和Ni被部分还原,Cu大量还原为Cu(0)。然后,计算模型确定了M 2+ N 4 -H 2 O为FeNC和CoNC中最活跃的中心,而Ni 1+ N 4相对于可逆氢电极,在-0.5和-0.6 V的考虑电势下,NiCl被认为是NiNC中最活跃的一个。可以从我们的密度泛函理论结果中合理化实验活性和选择性,特别是确定CO 2 * -和H *的结合能之间的差异作为对CO的选择性的描述指标。这种对活性和选择性的深入理解基于金属在原子分散的MN x位置上的eCO 2 RR的形态,提供了MNC催化剂针对eCO 2 RR的合理设计的指导原则,以用于其在高性能设备中的应用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号