当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Relative Solvent Exposure of the Alpha-Helix and Beta-Sheet in Water Determines the Initial Stages of Urea and Guanidinium Chloride-Induced Denaturation of Alpha/Beta Proteins.
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2019-10-11 , DOI: 10.1021/acs.jpcb.9b06859 Sridip Parui 1 , Biman Jana 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2019-10-11 , DOI: 10.1021/acs.jpcb.9b06859 Sridip Parui 1 , Biman Jana 1
Affiliation
|
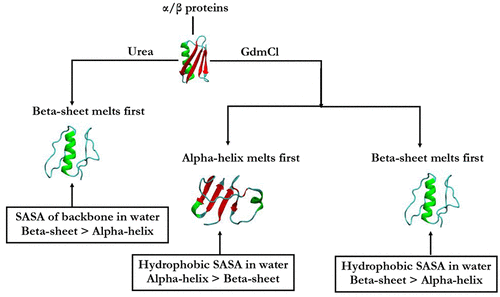
Among several denaturants, urea and guanidinium chloride (GdmCl) are the two strong and extensively used denaturants in unfolding experiments. However, the sequences of events in terms of secondary structure melting of several proteins in these two solvents have generated diverse results. A clear molecular understanding has still not been drawn to address the differential secondary structure specificity between urea and GdmCl. Here, we present a way to predict the possible unfolding route of α/β proteins, in terms of secondary structure melting, based on the observation of relative solvent exposure of the alpha-helix and beta-sheet of protein in water. We find that while beta-sheet always melts first in urea, a diverse preference is evident for GdmCl. Larger solvent exposure of the backbone of the beta-sheet helps the beta-sheet to make favorable hydrogen bonds with urea which in turn causes the initial melting of the beta-sheet of alpha/beta proteins in urea. On the other hand, because GdmCl has hydrophobic weakening ability through preferential interaction, the region which has comparatively more hydrophobic solvent exposure melts first in GdmCl. Urea- and GdmCl-induced initial unfolding pathways of the alpha/beta protein is thus determined by the relative solvent exposure of the alpha-helix and beta-sheet of protein in water. Therefore, detailed knowledge of relative solvent exposures in water can provide a hint of the possible unfolding pathway provided the mode of action of the solvent is known.
中文翻译:

Alpha-Helix和Beta-Sheet在水中的相对溶剂暴露确定了尿素和胍基氯化物诱导的Alpha / Beta蛋白变性的初始阶段。
在几种变性剂中,脲和氯化胍(GdmCl)是展开实验中的两种强力且广泛使用的变性剂。但是,就这两种溶剂中几种蛋白质的二级结构解链而言,事件序列产生了多种结果。对于尿素和GdmCl之间的二级结构差异,尚未有清晰的分子理解。在这里,我们根据观察到的蛋白质的α-螺旋和β-折叠的相对溶剂暴露量,提出了一种根据二级结构融化来预测α/β蛋白质可能展开途径的方法。我们发现,尽管β-片层始终总是首先在尿素中熔化,但对于GdmCl而言,显然存在多种多样的偏好。β-折叠骨架的较大溶剂暴露有助于β-折叠与尿素形成有利的氢键,进而导致尿素中的α/β蛋白的β-折叠最初融化。另一方面,由于GdmCl通过优先相互作用而具有疏水性减弱能力,因此疏水性溶剂暴露较多的区域首先在GdmCl中熔融。因此,尿素和GdmCl诱导的α/β蛋白质的初始展开路径由蛋白质的α-螺旋和β-折叠在水中的相对溶剂暴露量确定。因此,如果已知溶剂的作用方式,则有关在水中的相对溶剂暴露的详细知识可以提供可能的展开途径的提示。
更新日期:2019-10-12
中文翻译:

Alpha-Helix和Beta-Sheet在水中的相对溶剂暴露确定了尿素和胍基氯化物诱导的Alpha / Beta蛋白变性的初始阶段。
在几种变性剂中,脲和氯化胍(GdmCl)是展开实验中的两种强力且广泛使用的变性剂。但是,就这两种溶剂中几种蛋白质的二级结构解链而言,事件序列产生了多种结果。对于尿素和GdmCl之间的二级结构差异,尚未有清晰的分子理解。在这里,我们根据观察到的蛋白质的α-螺旋和β-折叠的相对溶剂暴露量,提出了一种根据二级结构融化来预测α/β蛋白质可能展开途径的方法。我们发现,尽管β-片层始终总是首先在尿素中熔化,但对于GdmCl而言,显然存在多种多样的偏好。β-折叠骨架的较大溶剂暴露有助于β-折叠与尿素形成有利的氢键,进而导致尿素中的α/β蛋白的β-折叠最初融化。另一方面,由于GdmCl通过优先相互作用而具有疏水性减弱能力,因此疏水性溶剂暴露较多的区域首先在GdmCl中熔融。因此,尿素和GdmCl诱导的α/β蛋白质的初始展开路径由蛋白质的α-螺旋和β-折叠在水中的相对溶剂暴露量确定。因此,如果已知溶剂的作用方式,则有关在水中的相对溶剂暴露的详细知识可以提供可能的展开途径的提示。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号