Applied Thermal Engineering ( IF 6.1 ) Pub Date : 2018-03-02 , DOI: 10.1016/j.applthermaleng.2018.02.069 Jian Li , Hanlin Gan , Yifeng Xu , Chaoyang Wang , FengLong Gu , Gang Wang
|
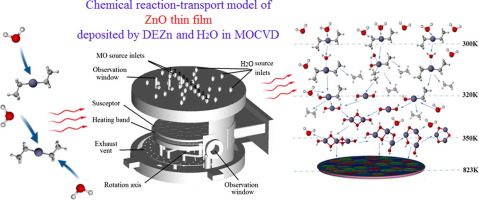
ZnO thin film has many uses as a semiconductor material. It can be fabricated by chemical vapor deposition with diethylzinc (DEZn) and water vapor. The present study employs density functional theory to examine reaction complexes of DEZn with 1–2 molecules of water, which may occur in the gas phase according to previous studies. The kinetic and thermodynamic data provide a better understanding of the ZnO deposition process. A computational fluid dynamics analysis was carried out using the kinetic parameters to simulate the deposition rate of ZnO in a reaction chamber, showing the dependence of the film growth rate on temperature. The simulation data agreed with the experimental one within 8%, proving the feasibility of the current chemical reaction-transport model of diethylzinc hydrolysis. Furthermore, the fields of the flow, the temperature, the multi-species transport, and the chemical reaction were analyzed. These insights could reveal the reaction kinetics of ZnO thin film fabrication in the metal-organic chemical vapor deposition chamber. The results show that the pathway involving nucleation and growth of oligomers from trimers, and ultimately particle formation, is consistent with the decreased growth rate at increasing temperatures. The maximum growth rate of ZnO film was obtained at 573–773 K when ZnO is grown by DEZn and H2O in a horizontal chamber rotating at high speed. When the temperature is above 773 K, parasitic reactions lead to a rapid decline in the ZnO deposition rate. These insights could reveal the reaction kinetics of ZnO thin film fabrication; and help with the reactor design, optimization, and process parameter adjustment in the metal-organic chemical vapor deposition process.
中文翻译:

立式MOCVD反应器中二乙基锌水解的化学反应-传输模型
ZnO薄膜作为半导体材料具有许多用途。可以使用二乙基锌(DEZn)和水蒸气通过化学气相沉积法制造。根据先前的研究,本研究采用密度泛函理论研究了DEZn与1-2个水分子的反应络合物,这可能在气相中发生。动力学和热力学数据提供了对ZnO沉积过程的更好理解。使用动力学参数进行了计算流体动力学分析,以模拟ZnO在反应室中的沉积速率,显示了膜生长速率对温度的依赖性。模拟数据与实验值吻合在8%以内,证明了现有的二乙基锌水解化学反应-传输模型的可行性。此外,流场,温度,分析了多种物种的运输和化学反应。这些见解可以揭示在有机金属化学气相沉积室内制备ZnO薄膜的反应动力学。结果表明,涉及三聚体的低聚物成核和生长以及最终形成颗粒的途径与温度升高时降低的生长速率一致。当用DEZn和H生长ZnO时,ZnO膜的最大生长速率在573–773 K处获得 与温度升高时降低的增长率一致。当用DEZn和H生长ZnO时,ZnO膜的最大生长速率在573–773 K处获得 与温度升高时降低的增长率一致。当用DEZn和H生长ZnO时,ZnO膜的最大生长速率在573–773 K处获得2 O在水平腔中高速旋转。当温度高于773 K时,寄生反应导致ZnO沉积速率迅速下降。这些见解可以揭示ZnO薄膜制造的反应动力学。并帮助金属有机化学气相沉积工艺中的反应器设计,优化和工艺参数调整。

































 京公网安备 11010802027423号
京公网安备 11010802027423号