Nature Cell Biology ( IF 17.3 ) Pub Date : 2019-10-01 , DOI: 10.1038/s41556-019-0397-z Adam Karoutas 1, 2 , Witold Szymanski 3 , Tobias Rausch 4 , Sukanya Guhathakurta 1, 2 , Eva A Rog-Zielinska 5 , Remi Peyronnet 5 , Janine Seyfferth 1 , Hui-Ru Chen 1, 2 , Rebecca de Leeuw 6 , Benjamin Herquel 1 , Hiroshi Kimura 7 , Gerhard Mittler 3 , Peter Kohl 5 , Ohad Medalia 6, 8 , Jan O Korbel 4 , Asifa Akhtar 1
|
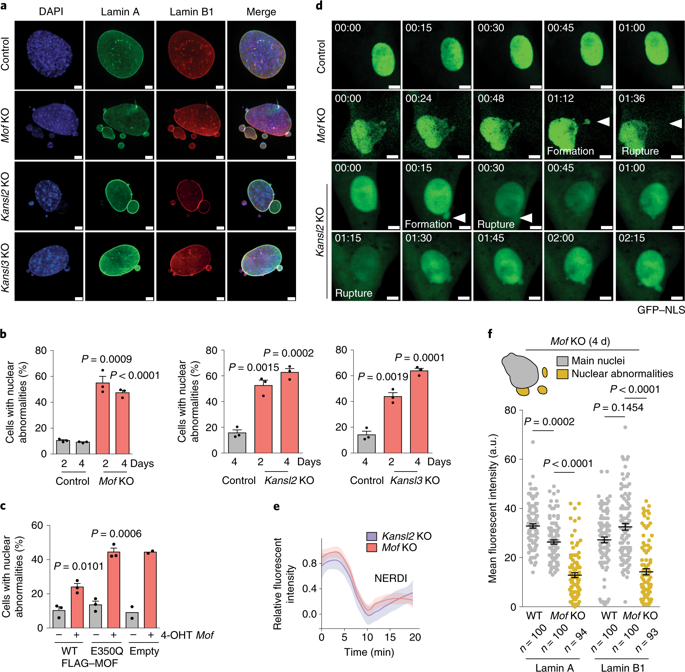
While nuclear lamina abnormalities are hallmarks of human diseases, their interplay with epigenetic regulators and precise epigenetic landscape remain poorly understood. Here, we show that loss of the lysine acetyltransferase MOF or its associated NSL-complex members KANSL2 or KANSL3 leads to a stochastic accumulation of nuclear abnormalities with genomic instability patterns including chromothripsis. SILAC-based MOF and KANSL2 acetylomes identified lamin A/C as an acetylation target of MOF. HDAC inhibition or acetylation-mimicking lamin A derivatives rescue nuclear abnormalities observed in MOF-deficient cells. Mechanistically, loss of lamin A/C acetylation resulted in its increased solubility, defective phosphorylation dynamics and impaired nuclear mechanostability. We found that nuclear abnormalities include EZH2-dependent histone H3 Lys 27 trimethylation and loss of nascent transcription. We term this altered epigenetic landscape “heterochromatin enrichment in nuclear abnormalities” (HENA). Collectively, the NSL-complex-dependent lamin A/C acetylation provides a mechanism that maintains nuclear architecture and genome integrity.
中文翻译:

NSL 复合体通过 lamin A/C 乙酰化维持核结构稳定性
虽然核层异常是人类疾病的标志,但它们与表观遗传调节因子的相互作用和精确的表观遗传景观仍然知之甚少。在这里,我们表明赖氨酸乙酰转移酶 MOF 或其相关的 NSL 复合物成员 KANSL2 或 KANSL3 的缺失会导致核异常的随机积累以及包括染色体碎裂在内的基因组不稳定模式。基于 SILAC 的 MOF 和 KANSL2 acetylomes 将核纤层蛋白 A/C 鉴定为 MOF 的乙酰化靶标。HDAC 抑制或模拟乙酰化的核纤层蛋白 A 衍生物可挽救在 MOF 缺陷细胞中观察到的核异常。从机制上讲,核纤层蛋白 A/C 乙酰化的缺失导致其溶解度增加、磷酸化动力学缺陷和核机械稳定性受损。我们发现核异常包括 EZH2 依赖性组蛋白 H3 Lys 27 三甲基化和新生转录缺失。我们将这种改变的表观遗传景观称为“核异常中的异染色质富集”(HENA)。总的来说,NSL 复合物依赖性核纤层蛋白 A/C 乙酰化提供了一种维持核结构和基因组完整性的机制。






























 京公网安备 11010802027423号
京公网安备 11010802027423号