当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preparation of 2-(2H-Tetrazol-2-yl)benzoic Acids via Regioselective Cu(I) Catalyzed N2 Arylation of Tetrazole
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2019-10-14 , DOI: 10.1021/acs.oprd.9b00243 Zhiguo Jake Song 1 , Peter Maligres 1 , Carmela Molinaro 1 , Guy Humphrey 1 , Jeff Fritzen 1 , Jonathan Wilson 1 , Yonggang Chen 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2019-10-14 , DOI: 10.1021/acs.oprd.9b00243 Zhiguo Jake Song 1 , Peter Maligres 1 , Carmela Molinaro 1 , Guy Humphrey 1 , Jeff Fritzen 1 , Jonathan Wilson 1 , Yonggang Chen 1
Affiliation
|
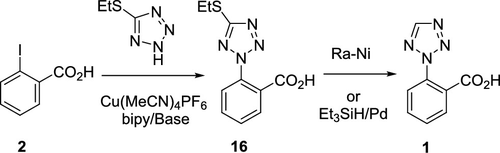
2-(2H-Tetrazol-2-yl)benzoic acid 1 and analogs were prepared via Cu(I) catalyzed C–N coupling of 2-iodo or 2-bromo benzoic acids with 5-(ethylthio)-1H-tetrazole followed by reductive cleavage of the thioether bond. The C–N coupling was regioselective toward the N2-position on tetrazole. The scope of this methodology was demonstrated on a series of 2-halobenzoic acid substrates in moderate yields.
中文翻译:

区域选择性Cu(I)催化四唑的N2芳基化反应制备2-(2 H-替他唑-2-基)苯甲酸
2-(2 H-替他唑-2-基)苯甲酸1及其类似物是通过Cu(I)催化2-碘或2-溴苯甲酸与5-(乙硫基)-1 H-四唑的C–N偶联制备的然后还原性裂解硫醚键。C–N偶联对四唑的N2-位置具有区域选择性。在一系列2-卤代苯甲酸底物上以中等收率证明了该方法的范围。
更新日期:2019-10-14
中文翻译:

区域选择性Cu(I)催化四唑的N2芳基化反应制备2-(2 H-替他唑-2-基)苯甲酸
2-(2 H-替他唑-2-基)苯甲酸1及其类似物是通过Cu(I)催化2-碘或2-溴苯甲酸与5-(乙硫基)-1 H-四唑的C–N偶联制备的然后还原性裂解硫醚键。C–N偶联对四唑的N2-位置具有区域选择性。在一系列2-卤代苯甲酸底物上以中等收率证明了该方法的范围。






























 京公网安备 11010802027423号
京公网安备 11010802027423号