Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evaluation of a Recombinant Newcastle Disease Virus Expressing Human IL12 against Human Breast Cancer.
Scientific Reports ( IF 3.8 ) Pub Date : 2019-09-30 , DOI: 10.1038/s41598-019-50222-z
Zahiah Mohamed Amin 1 , Muhamad Alhapis Che Ani 2 , Sheau Wei Tan 1 , Swee Keong Yeap 3 , Noorjahan Banu Alitheen 1, 2 , Syed Umar Faruq Syed Najmuddin 1 , Jeevanathan Kalyanasundram 2 , Soon Choy Chan 4 , Abhi Veerakumarasivam 5, 6 , Suet Lin Chia 1, 2 , Khatijah Yusoff 2, 6
Scientific Reports ( IF 3.8 ) Pub Date : 2019-09-30 , DOI: 10.1038/s41598-019-50222-z
Zahiah Mohamed Amin 1 , Muhamad Alhapis Che Ani 2 , Sheau Wei Tan 1 , Swee Keong Yeap 3 , Noorjahan Banu Alitheen 1, 2 , Syed Umar Faruq Syed Najmuddin 1 , Jeevanathan Kalyanasundram 2 , Soon Choy Chan 4 , Abhi Veerakumarasivam 5, 6 , Suet Lin Chia 1, 2 , Khatijah Yusoff 2, 6
Affiliation
|
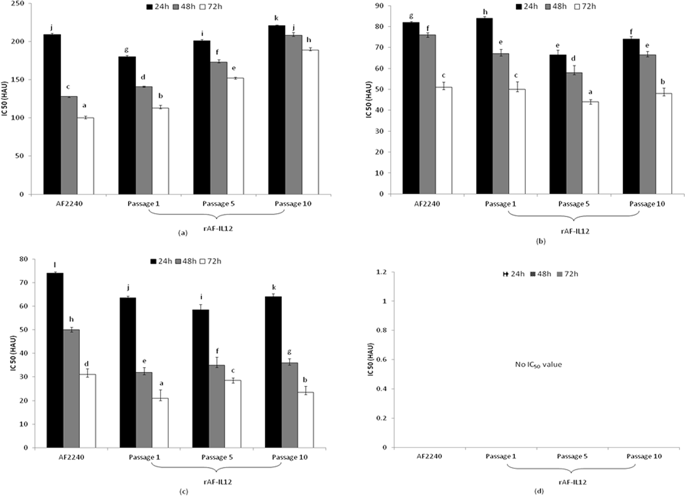
The Newcastle disease virus (NDV) strain AF2240 is an avian avulavirus that has been demonstrated to possess oncolytic activity against cancer cells. However, to illicit a greater anti-cancer immune response, it is believed that the incorporation of immunostimulatory genes such as IL12 into a recombinant NDV backbone will enhance its oncolytic effect. In this study, a newly developed recombinant NDV that expresses IL12 (rAF-IL12) was tested for its safety, stability and cytotoxicity. The stability of rAF-IL12 was maintained when passaged in specific pathogen free (SPF) chicken eggs from passage 1 to passage 10; with an HA titer of 29. Based on the results obtained from the MTT cytotoxic assay, rAF-IL12 was determined to be safe as it only induced cytotoxic effects against normal chicken cell lines and human breast cancer cells while sparing normal cells. Significant tumor growth inhibition (52%) was observed in the rAF-IL12-treated mice. The in vivo safety profile of rAF-IL12 was confirmed through histological observation and viral load titer assay. The concentration and presence of the expressed IL12 was quantified and verified via ELISA assay. In summary, rAF-IL12 was proven to be safe, selectively replicating in chicken and cancer cells and was able to maintain its stability throughout several passages; thus enhancing its potential as an anti-breast cancer vaccine.
中文翻译:

表达针对人乳腺癌的表达人IL12的重组新城疫病毒的评估。
新城疫病毒(NDV)株AF2240是一种禽肺泡病毒,已被证明对癌细胞具有溶瘤活性。然而,为了非法获得更大的抗癌免疫应答,相信将免疫刺激基因例如IL12掺入重组NDV主链将增强其溶瘤作用。在这项研究中,测试了新开发的表达IL12(rAF-IL12)的重组NDV的安全性,稳定性和细胞毒性。从第1代到第10代,在无特定病原体(SPF)的鸡蛋中传代时,rAF-IL12的稳定性得以保持;HA滴度为29。根据MTT细胞毒性试验获得的结果,rAF-IL12被确定为安全的,因为它仅诱导针对正常鸡细胞系和人乳腺癌细胞的细胞毒性作用,而保留正常细胞。在用rAF-IL12治疗的小鼠中观察到显着的肿瘤生长抑制(52%)。通过组织学观察和病毒载量滴度测定法证实了rAF-IL12的体内安全性。定量表达的IL12的浓度和存在,并通过ELISA分析进行验证。总之,事实证明,rAF-IL12是安全的,可以在鸡和癌细胞中选择性复制,并且能够在数个传代中保持其稳定性。因此增强了其作为抗乳腺癌疫苗的潜力。通过组织学观察和病毒载量滴度测定法证实了rAF-IL12的体内安全性。定量表达的IL12的浓度和存在,并通过ELISA分析进行验证。总之,事实证明,rAF-IL12是安全的,可以在鸡和癌细胞中选择性复制,并且能够在数个传代中保持其稳定性。因此增强了其作为抗乳腺癌疫苗的潜力。通过组织学观察和病毒载量滴度测定证实了rAF-IL12的体内安全性。定量表达的IL12的浓度和存在,并通过ELISA分析进行验证。总之,事实证明,rAF-IL12是安全的,可以在鸡和癌细胞中选择性复制,并且能够在数个传代中保持其稳定性。因此增强了其作为抗乳腺癌疫苗的潜力。
更新日期:2019-09-30
中文翻译:

表达针对人乳腺癌的表达人IL12的重组新城疫病毒的评估。
新城疫病毒(NDV)株AF2240是一种禽肺泡病毒,已被证明对癌细胞具有溶瘤活性。然而,为了非法获得更大的抗癌免疫应答,相信将免疫刺激基因例如IL12掺入重组NDV主链将增强其溶瘤作用。在这项研究中,测试了新开发的表达IL12(rAF-IL12)的重组NDV的安全性,稳定性和细胞毒性。从第1代到第10代,在无特定病原体(SPF)的鸡蛋中传代时,rAF-IL12的稳定性得以保持;HA滴度为29。根据MTT细胞毒性试验获得的结果,rAF-IL12被确定为安全的,因为它仅诱导针对正常鸡细胞系和人乳腺癌细胞的细胞毒性作用,而保留正常细胞。在用rAF-IL12治疗的小鼠中观察到显着的肿瘤生长抑制(52%)。通过组织学观察和病毒载量滴度测定法证实了rAF-IL12的体内安全性。定量表达的IL12的浓度和存在,并通过ELISA分析进行验证。总之,事实证明,rAF-IL12是安全的,可以在鸡和癌细胞中选择性复制,并且能够在数个传代中保持其稳定性。因此增强了其作为抗乳腺癌疫苗的潜力。通过组织学观察和病毒载量滴度测定法证实了rAF-IL12的体内安全性。定量表达的IL12的浓度和存在,并通过ELISA分析进行验证。总之,事实证明,rAF-IL12是安全的,可以在鸡和癌细胞中选择性复制,并且能够在数个传代中保持其稳定性。因此增强了其作为抗乳腺癌疫苗的潜力。通过组织学观察和病毒载量滴度测定证实了rAF-IL12的体内安全性。定量表达的IL12的浓度和存在,并通过ELISA分析进行验证。总之,事实证明,rAF-IL12是安全的,可以在鸡和癌细胞中选择性复制,并且能够在数个传代中保持其稳定性。因此增强了其作为抗乳腺癌疫苗的潜力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号