当前位置:
X-MOL 学术
›
Environ. Pollut.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Distribution, transformation and toxicity evaluation of 2,6-Di-tert-butyl-hydroxytotulene in aquatic environment.
Environmental Pollution ( IF 7.6 ) Pub Date : 2019-09-30 , DOI: 10.1016/j.envpol.2019.113330 Yan Wang 1 , Lin He 2 , Guochun Lv 1 , Wen Liu 1 , Jiashuo Liu 1 , Xiaohui Ma 1 , Xiaomin Sun 1
Environmental Pollution ( IF 7.6 ) Pub Date : 2019-09-30 , DOI: 10.1016/j.envpol.2019.113330 Yan Wang 1 , Lin He 2 , Guochun Lv 1 , Wen Liu 1 , Jiashuo Liu 1 , Xiaohui Ma 1 , Xiaomin Sun 1
Affiliation
|
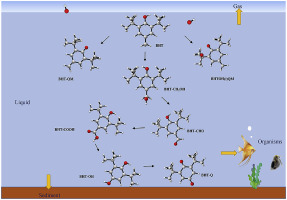
2,6-Di-tert-butyl-hydroxytotulene (BHT), as a significant synthetic phenolic antioxidant (SPA), has received increasing attention in the environmental field. In the present study, the BHT is confirmed to be mainly distributed in the liquid phase in the environment base on the Aspen PLUS simulation results. The mechanism and kinetics of BHT transformation initiated by OH radicals were conducted in aquatic environment using density functional theory (DFT) method. Briefly, seven initiation reactions and three detailed transformation pathways of BHT were reported. The H atoms in the t-butyl and methyl group were found more favorable to be abstracted. The C1 site of the BHT was susceptible to addition by OH radicals. Rate constants of different initial reactions were calculated and they were inhibited by temperature rise. Meanwhile, the acute and chronic toxicities of BHT and its metabolites were evaluated at three different trophic levels using the ECOSAR program. During the degradation process, the toxicities of these metabolites gradually decreased, but the toxicities of the final product 2,6-di-tert-butyl-2,5-cyclohexadien-1,4-dione (BHT-Q) were significantly increased. These results could help to reveal the transformation mechanism and risk assessment of BHT in aquatic environment, and further design the experimental and industrial applications of SPAs.
中文翻译:

2,6-二叔丁基-羟基甲苯在水生环境中的分布,转化和毒性评价。
2,6-二叔丁基-羟基甲苯(BHT),作为一种重要的合成酚类抗氧化剂(SPA),在环境领域受到越来越多的关注。在本研究中,根据Aspen PLUS模拟结果,已确认BHT主要分布在环境中的液相中。利用密度泛函理论(DFT)方法在水生环境中进行了由OH自由基引发的BHT转化的机理和动力学。简要地,报道了BHT的七个起始反应和三个详细的转化途径。发现叔丁基和甲基中的H原子更易于被提取。BHT的C1位易被OH基加成。计算了不同初始反应的速率常数,它们受到温度升高的抑制。同时,使用ECOSAR程序在三种不同营养水平下评估了BHT及其代谢产物的急性和慢性毒性。在降解过程中,这些代谢物的毒性逐渐降低,但最终产物2,6-二叔丁基-2,5-环己二烯-1,4-二酮(BHT-Q)的毒性显着增加。这些结果有助于揭示水生环境中BHT的转化机理和风险评估,并进一步设计SPA的实验和工业应用。4-二酮(BHT-Q)明显增加。这些结果有助于揭示水生环境中BHT的转化机理和风险评估,并进一步设计SPA的实验和工业应用。4-二酮(BHT-Q)明显增加。这些结果有助于揭示水生环境中BHT的转化机理和风险评估,并进一步设计SPA的实验和工业应用。
更新日期:2019-09-30
中文翻译:

2,6-二叔丁基-羟基甲苯在水生环境中的分布,转化和毒性评价。
2,6-二叔丁基-羟基甲苯(BHT),作为一种重要的合成酚类抗氧化剂(SPA),在环境领域受到越来越多的关注。在本研究中,根据Aspen PLUS模拟结果,已确认BHT主要分布在环境中的液相中。利用密度泛函理论(DFT)方法在水生环境中进行了由OH自由基引发的BHT转化的机理和动力学。简要地,报道了BHT的七个起始反应和三个详细的转化途径。发现叔丁基和甲基中的H原子更易于被提取。BHT的C1位易被OH基加成。计算了不同初始反应的速率常数,它们受到温度升高的抑制。同时,使用ECOSAR程序在三种不同营养水平下评估了BHT及其代谢产物的急性和慢性毒性。在降解过程中,这些代谢物的毒性逐渐降低,但最终产物2,6-二叔丁基-2,5-环己二烯-1,4-二酮(BHT-Q)的毒性显着增加。这些结果有助于揭示水生环境中BHT的转化机理和风险评估,并进一步设计SPA的实验和工业应用。4-二酮(BHT-Q)明显增加。这些结果有助于揭示水生环境中BHT的转化机理和风险评估,并进一步设计SPA的实验和工业应用。4-二酮(BHT-Q)明显增加。这些结果有助于揭示水生环境中BHT的转化机理和风险评估,并进一步设计SPA的实验和工业应用。






























 京公网安备 11010802027423号
京公网安备 11010802027423号