Journal of CO2 Utilization ( IF 7.2 ) Pub Date : 2019-09-28 , DOI: 10.1016/j.jcou.2019.09.015 D. Hospital-Benito , J. Lemus , R. Santiago , J. Palomar
|
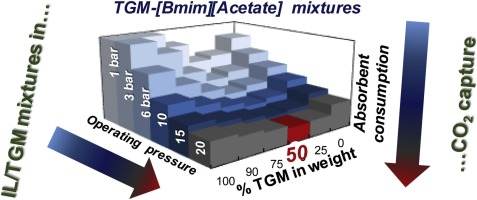
The mixture of ionic liquids (ILs) with a low viscous solvent such as tetraglyme (TGM) may be interesting to reach new absorbent formulations with improved performance in CO2 capture technologies. In this work, CO2 absorption by IL/TGM mixtures was analyzed by equilibrium and kinetic gravimetric measurements and packed column simulations in Aspen Plus. Four ILs with remarkable different absorbent properties were employed. The ILs 1-butyl-methylimidazolium tricyanomethanide ([Bmim][TCM]) and 1-butyl-methylimidazolium methylsulfate ([Bmim][MeSO4]) present CO2 physical absorption, with remarkably different viscosity values. On the other hand, the ILs trihexyltetradecylphosphonium 2-cyanopyrrolide ([P66614][CNPyr]) and1-butyl-methylimidazolium acetate ([Bmim][Acetate]) were selected as CO2 chemical absorbents, being the former significantly less viscous. CO2 absorption experiments were carried out in a magnetic suspension microbalance using a wide composition range of IL/TGM mixtures, measured at 303 K and at different pressures, from 1 up to 20 bar. The results were analyzed considering thermodynamics (CO2 solubility in IL/TGM) and kinetics (CO2 diffusivity in IL/TGM) features. ILs with physical absorption presented higher CO2 solubilities and diffusivities when increasing TGM composition. In fact, the neat TGM shows higher CO2 physical absorption capacity and rate than IL/TGM mixtures at any studied opeating conditions. In contrast, ILs with CO2 chemical absorption generally present higher CO2 absorption capacity than TGM/IL mixtures, but a certain amount of TGM improves drastically the CO2 absortion kinetics with a slight decrease of the solubility. The simulation analysis of IL/TGM performance in the absorption column reveals that: i) TGM is better physical absorbent for CO2 capture than IL/TGM mixtures and neat ILs; and ii) it is possible to select finest IL/TGM mixtures which minimize the solvent and energy expenses in CO2 capture by chemical absorption.
中文翻译:

离子液体和四甘醇二甲醚混合物对CO 2捕集的热力学和动力学评估
离子液体(ILs)与低粘度溶剂(如四甘醇二甲醚(TGM))的混合物可能很有趣,以生产具有改进的CO 2捕集技术性能的新型吸收剂配方。在这项工作中,通过Aspen Plus中的平衡和动力学重量分析以及填充柱模拟分析了IL / TGM混合物对CO 2的吸收。使用了四种具有明显不同吸收特性的IL。IL的1-丁基-甲基咪唑三氰胺([Bmim] [TCM])和1-丁基-甲基咪唑甲基硫酸盐([Bmim] [MeSO 4 ])表现为CO 2物理吸收,粘度值明显不同。另一方面,白介素三氰基十四烷基phosph2-氰基吡咯化物([P 66614选择[CNPyr])和1-丁基甲基咪唑乙酸盐([Bmim] [乙酸盐])作为CO 2化学吸收剂,前者的黏度明显较低。在磁悬浮微量天平中使用宽范围的IL / TGM混合物组成进行了CO 2吸收实验,在303 K和1至20 bar的不同压力下进行了测量。分析结果时要考虑热力学(在IL / TGM中的CO 2溶解度)和动力学(在IL / TGM中的CO 2扩散性)特征。当增加TGM组成时,具有物理吸收的IL表现出更高的CO 2溶解度和扩散性。实际上,纯净的TGM表现出更高的CO 2在任何研究的使用条件下,其物理吸收能力和吸收速率均比IL / TGM混合物高。相反,具有CO 2化学吸收的IL通常比TGM / IL混合物具有更高的CO 2吸收能力,但是一定量的TGM可以显着改善CO 2吸收动力学,但溶解度略有降低。在吸收塔中对IL / TGM性能的仿真分析表明:i)与IL / TGM混合物和纯净的IL相比,TGM对CO 2捕获的物理吸收性更好。ii)可以选择最优质的IL / TGM混合物,以最大程度地减少通过化学吸收捕集CO 2所消耗的溶剂和能量。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号