当前位置:
X-MOL 学术
›
Colloids Surf. A Physicochem. Eng. Aspects
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper sulfide flotation under acidic conditions using a xanthogen formate compound as collector: adsorption studies and experimental design approach
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 4.9 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.colsurfa.2019.124032
D.M. Ávila-Márquez , A. Blanco-Flores , I.A. Reyes-Domínguez , H.P. Toledo-Jaldin , J. Aguilar-Carrillo , R. Cruz-Gaona
Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 4.9 ) Pub Date : 2020-01-01 , DOI: 10.1016/j.colsurfa.2019.124032
D.M. Ávila-Márquez , A. Blanco-Flores , I.A. Reyes-Domínguez , H.P. Toledo-Jaldin , J. Aguilar-Carrillo , R. Cruz-Gaona
|
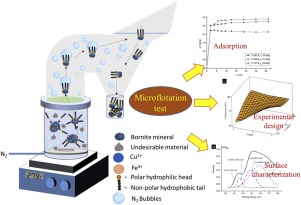
Abstract Under acidic conditions, isobutyl xanthogen ethyl formate was used as a collector compound in the flotation process for Cu recovery from the mineral bornite (Cu5FeS4). The mineral sample was characterized by X-Ray Diffraction (XRD), Fourier-transform infrared spectroscopy (FT-IR), Scanning Electron Microscopy (SEM) and X-Ray Photoelectron Spectroscopy (XPS) techniques. Also found in lower proportions were chalcopyrite and quartz. The bornite presented a rough and porous surface. The adsorption study included kinetics, thermodynamics, and isotherm tests. The equilibrium time at pH 2 was 15 min. The kinetics model suggest the adsorption can be described by two processes, both related to the formation of a chelate. Thermodynamically, the collector adsorption process is favored at room temperature, whereas the process is not favored at lower temperatures. The adsorption capacity was 8.8 mg g−1 achieved by a combination of mechanisms on a heterogeneous surface. Under local optimized conditions, the highest Cu recovery (96.98 %) was determined from an experimental design for an initial collector concentration of 20 mg L−1, a flotation time of 3 min, and pH = 2. Contact time of the collector with the mineral surface represents one of the most important variables in the flotation process. In addition, the combinations between pH-tf, Ci-tf, and pH-Ci-tf variables have the most significant effect on Cu recovery percentage. The collector can be used satisfactorily at lower pH values. Although the xanthogen formate functional group acted on both the Fe and Cu ions in the mineral, it was observed that the collector has a higher affinity for Cu ions compared with Fe ions.
中文翻译:

使用黄原甲酸盐化合物作为捕收剂在酸性条件下浮选硫化铜:吸附研究和实验设计方法
摘要 在酸性条件下,甲酸异丁基黄原酸乙酯在浮选过程中用作捕收剂化合物,用于从矿物斑铜矿 (Cu5FeS4) 中回收铜。通过 X 射线衍射 (XRD)、傅里叶变换红外光谱 (FT-IR)、扫描电子显微镜 (SEM) 和 X 射线光电子能谱 (XPS) 技术对矿物样品进行了表征。还发现比例较低的还有黄铜矿和石英。斑铜矿表面粗糙且多孔。吸附研究包括动力学、热力学和等温线测试。pH 2 时的平衡时间为 15 分钟。动力学模型表明吸附可以通过两个过程来描述,这两个过程都与螯合物的形成有关。热力学上,常温下有利于收集器吸附过程,而该过程在较低温度下不受欢迎。通过异质表面上的机制组合实现的吸附容量为 8.8 mg g-1。在局部优化条件下,最高铜回收率 (96.98 %) 由实验设计确定,初始捕收剂浓度为 20 mg L-1,浮选时间为 3 分钟,pH = 2。捕收剂与捕收剂的接触时间矿物表面是浮选过程中最重要的变量之一。此外,pH-tf、Ci-tf 和 pH-Ci-tf 变量之间的组合对铜回收率的影响最为显着。收集器可以在较低的 pH 值下令人满意地使用。尽管黄原甲酸官能团同时作用于矿物中的 Fe 和 Cu 离子,
更新日期:2020-01-01
中文翻译:

使用黄原甲酸盐化合物作为捕收剂在酸性条件下浮选硫化铜:吸附研究和实验设计方法
摘要 在酸性条件下,甲酸异丁基黄原酸乙酯在浮选过程中用作捕收剂化合物,用于从矿物斑铜矿 (Cu5FeS4) 中回收铜。通过 X 射线衍射 (XRD)、傅里叶变换红外光谱 (FT-IR)、扫描电子显微镜 (SEM) 和 X 射线光电子能谱 (XPS) 技术对矿物样品进行了表征。还发现比例较低的还有黄铜矿和石英。斑铜矿表面粗糙且多孔。吸附研究包括动力学、热力学和等温线测试。pH 2 时的平衡时间为 15 分钟。动力学模型表明吸附可以通过两个过程来描述,这两个过程都与螯合物的形成有关。热力学上,常温下有利于收集器吸附过程,而该过程在较低温度下不受欢迎。通过异质表面上的机制组合实现的吸附容量为 8.8 mg g-1。在局部优化条件下,最高铜回收率 (96.98 %) 由实验设计确定,初始捕收剂浓度为 20 mg L-1,浮选时间为 3 分钟,pH = 2。捕收剂与捕收剂的接触时间矿物表面是浮选过程中最重要的变量之一。此外,pH-tf、Ci-tf 和 pH-Ci-tf 变量之间的组合对铜回收率的影响最为显着。收集器可以在较低的 pH 值下令人满意地使用。尽管黄原甲酸官能团同时作用于矿物中的 Fe 和 Cu 离子,

































 京公网安备 11010802027423号
京公网安备 11010802027423号